Hematology
Explore our range of hematology resources, including expert opinion, recent guideline updates, information on emerging treatments, and more.
Medical conditions
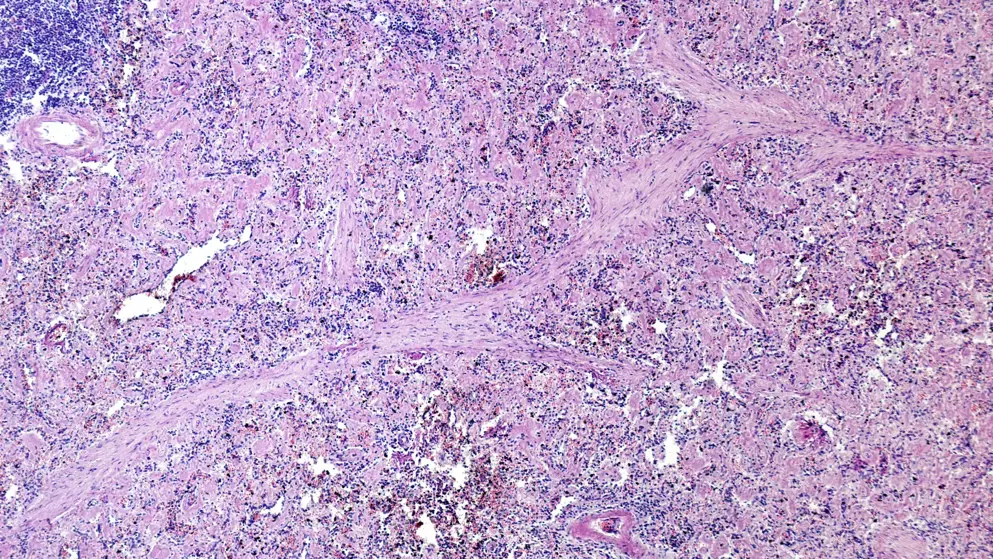
AL amyloidosis
Patient insights and expert opinion
Coagulopathy and bleeding disorders
Latest news, insights, and guideline updates
HLH/MAS
Key disease features and expert insights
HIV
Guidelines and considerations for management
Leukemia
Latest news, insights, and guideline updates
Lymphoma
Earn CME credit, plus expert congress highlights
Multiple myeloma
Latest news, insights, and guideline updates
Venous thromboembolism
Expert podcast and congress highlights
AL amyloidosis
Hear the latest strategies to support timely diagnosis and optimal management of this difficult-to-identify rare disease with our interactive patient journey and expert podcast series.
Bleeding risk after DOAC initiation
From ISTH 2024, read results from a study investigating time to bleeding after DOAC initiation for venous thromboembolism and atrial fibrillation.