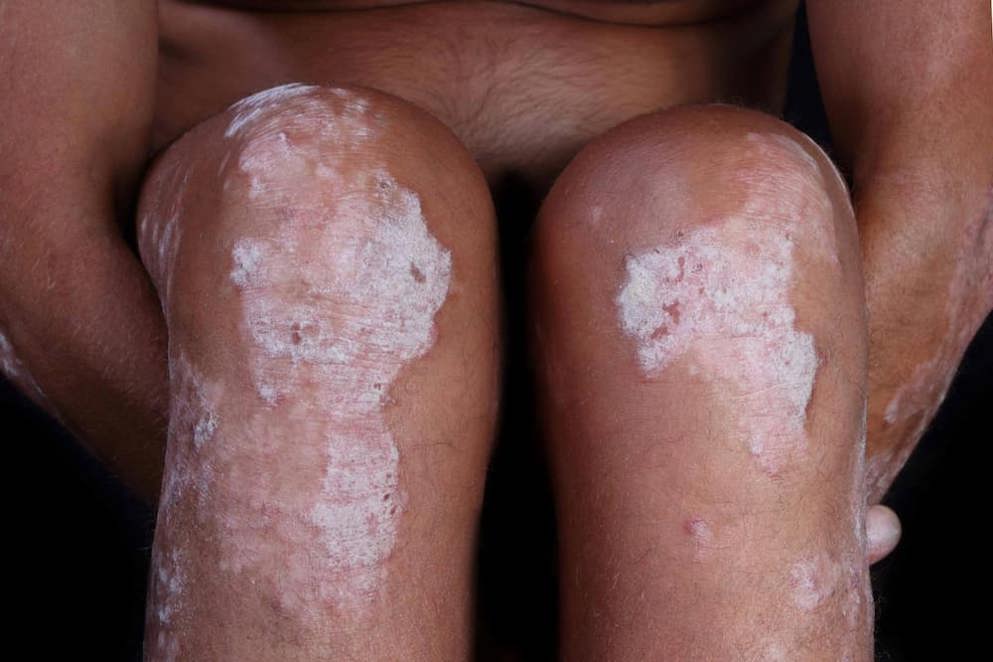
Novartis Cosentyx receives FDA approval for treatment of children and adolescents with moderate to severe plaque psoriasis.
“Treating moderate to severe plaque psoriasis in children can be complicated, as we need to balance the ability of a treatment to provide symptom relief while considering the safety profile as the top priority,” said John Browning, MD, FAAD, FAAP, MBA, clinical trial investigator, Adjunct Associate Professor of Pediatrics and Dermatology at the University of Texas Health, San Antonio. “In the pediatric pivotal study, the majority of patients treated with Cosentyx were able to achieve clear or almost clear skin with a safety profile consistent with previous clinical trials in adults. Due to the systemic nature of the disease, Cosentyx is a welcome addition as a treatment option for families dealing with this challenging condition.” The approved pediatric dosing for Cosentyx is 75 mg or 150 mg depending on the child’s weight at the time of dosing and is administered by subcutaneous injection every four weeks after an initial loading regimen. After initial counseling and proper training in injection technique, Cosentyx can be administered by an adult caregiver outside of a healthcare provider’s office via a single-dose prefilled syringe or Sensoready pen.