News
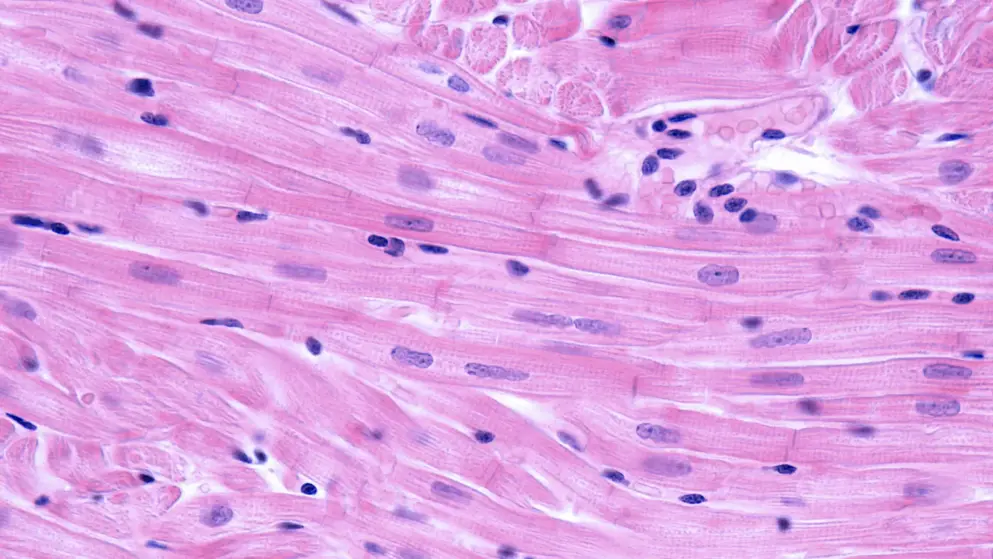
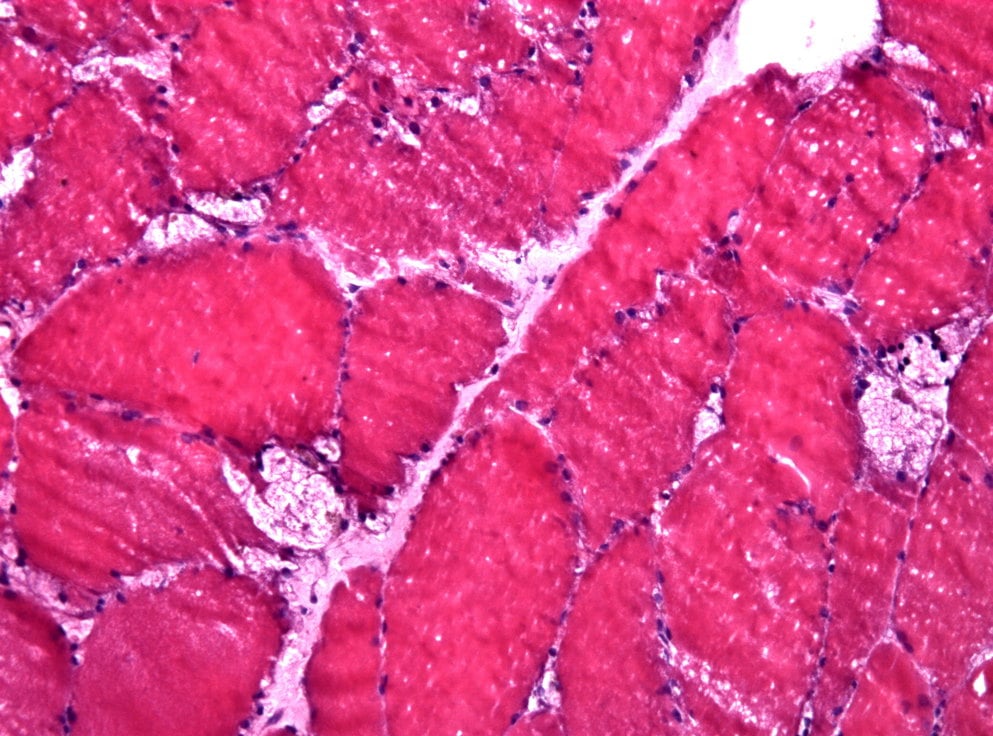
Sanofi’s avalglucosidase alfa shows clinically meaningful improvement in critical manifestations of late-onset Pompe disease.
Sanofi’s investigational enzyme replacement therapy (ERT), avalglucosidase alfa, showed clinically meaningful improvement in critical manifestations (respiratory impairment and decreased mobility) of late-onset Pompe disease (LOPD) according to results from the Phase III trial presented at a Sanofi-hosted scientific session.
Avalglucosidase alfa met the primary endpoint demonstrating non-inferiority in improving respiratory function compared to alglucosidase alfa (standard of care) in patients with LOPD. These data will form the basis for global regulatory submissions anticipated in the second half of this year. The FDA)has granted Breakthrough Therapy and Fast Track designations to avalglucosidase alfa for the treatment of patients with Pompe disease.
The trial primary endpoint evaluated the change in respiratory muscle function using percent-predicted forced vital capacity (FVC) in the upright position. Patients treated with avalglucosidase alfa had a 2.4-point greater improvement in percent-predicted FVC compared to patients treated with standard of care (95% CI, -0.13 / 4.99), a numerical improvement in respiratory function that surpassed the study-designed measure of non-inferiority (p=0.0074). The primary endpoint was also measured for superiority. Superiority statistical significance was not achieved for the avalglucosidase alfa arm (p=0.0626). As a result, per the hierarchy of the study protocol, formal statistical testing for all secondary endpoints was not conducted. A key secondary endpoint in the trial measured mobility with the 6-minute walk test (6MWT). Patients treated with avalglucosidase alfa walked 30 meters farther (95% CI, 1.33 / 58.69) than patients treated with standard of care. Other secondary endpoints assessed respiratory muscle strength, motor function, and quality of life.
Also presented were results from a pre-specified preliminary analysis evaluating percent-predicted FVC and 6MWT in those patients who switched (switch patients) at 49 weeks from standard of care to avalglucosidase alfa for the open-label extension period of the trial. Due to sequential enrollment, preliminary analysis results at the time of data presentation were available at 97 weeks for 20 out of 49 switch patients for percent-predicted FVC, and 21 out of 49 switch patients for 6MWT. In these switch patients, avalglucosidase alfa demonstrated a 0.15-point improvement in FVC (95% CI, -1.95 / 2.25) and a 23.32-meter improvement in 6MWT (95% CI, -3.87 / 50.51).
The safety profile of avalglucosidase alfa was found to be comparable to standard of care. Over the double-blinded 49-week period, 44 patients in the avalglucosidase alfa arm and 45 patients in the standard of care arm experienced an adverse event(s) (AEs). There were 6 patients with severe AEs in the avalglucosidase alfa arm and 7 patients in the standard of care arm. Fewer patients presented with serious adverse events (SAEs) in the avalglucosidase alfa arm (8 patients, including 1 patient with potentially treatment-related SAEs) compared to the standard of care arm (12 patients, including 3 patients with potentially treatment-related SAEs). In the standard of care arm of the trial, 4 patients had AEs leading to study withdrawal and 1 patient died due to an SAE of acute myocardial infarction (unrelated to treatment). In the avalglucosidase alfa arm, there were no patient discontinuations or deaths. Fewer patients in the avalglucosidase alfa arm (25.5%) experienced at least one protocol-defined infusion-associated reaction compared to the alglucosidase alfa arm (32.7%). Immunogenicity data is being analysed and will be presented at a future medical congress or publication..
Condition: Pompe Disease
Type: drug