FDA approves Ngenla to treat human growth hormone analog indicated for treatment of pediatric patients aged three years and older who have growth failure due to inadequate secretion of endogenous growth hormone
Pfizer Inc and OPKO Health Inc. announced that the FDA has approved Ngenla (somatrogon-ghla), a once-weekly, human growth hormone analog indicated for treatment of pediatric patients aged three years and older who have growth failure due to inadequate secretion of endogenous growth hormone
Ngenla is expected to become available for U.S. prescribing in August 2023.
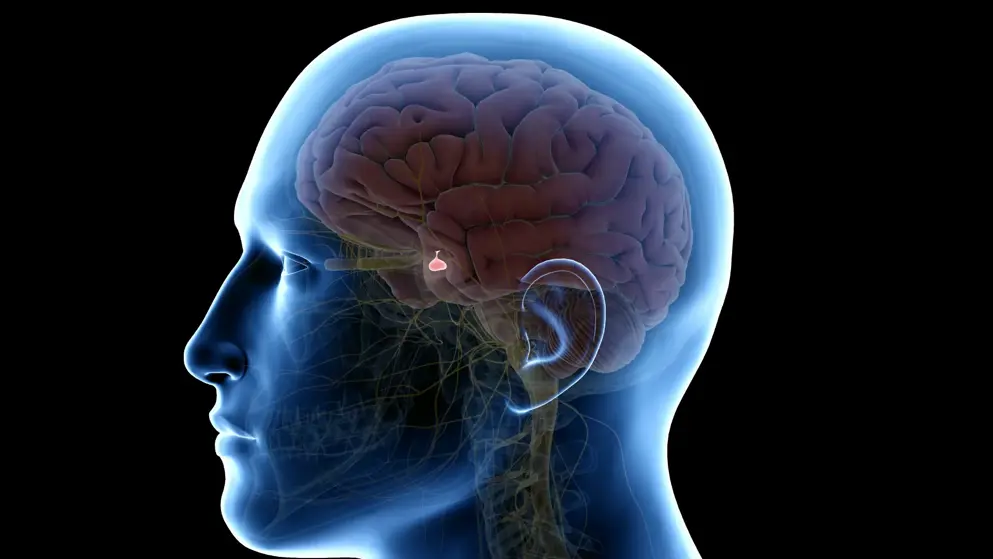
Growth hormone deficiency (GHD) is a rare disease characterized by the inadequate secretion of the growth hormone somatropin from the pituitary gland, affecting one in approximately 4,000 to 10,000 children. Without treatment, children will have persistent growth attenuation, a very short height in adulthood, and puberty may be delayed. Children living with GHD may also experience challenges in relation to their physical health and mental well-being.
The FDA approval is supported by results from a multi-center, randomized, open-label, active-controlled Phase III study which evaluated the safety and efficacy of Ngenla when administered once-weekly compared to once-daily somatropin. The study met its primary endpoint of Ngenla non-inferiority compared to somatropin, as measured by annual height velocity at 12 months. Ngenla was generally well tolerated in the study and had a safety profile comparable to somatropin.
The approval of Ngenla will be significant for children with growth hormone deficiency in the U.S. It holds potential to reduce the treatment burden that can come with daily growth hormone injections,” said Joel Steelman, M.D., Pediatric Endocrinologist, Cook Children’s Health Care System. “As a new, longer-acting option that has the ability to reduce treatment frequency from daily to weekly, Ngenla could become an important treatment option that can improve adherence for children being treated for growth hormone deficiency.”