News
Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved Vyvgart intravenous infusion.
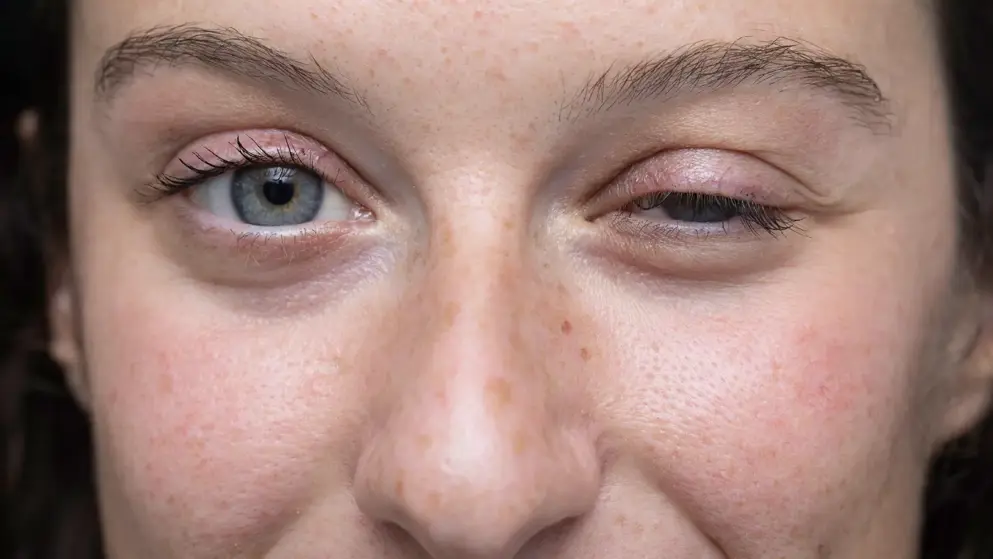
Argenx announced that Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved Vyvgart (efgartigimod alfa) intravenous infusion for the treatment of adult patients with generalized myasthenia gravis (gMG) who do not have sufficient response to steroids or non-steroidal immunosuppressive therapies (ISTs).
Vyvgart is the first-and-only neonatal Fc receptor (FcRn) blocker approved in Japan.
Generalized myasthenia gravis is a rare and chronic neuromuscular disease characterized by debilitating and potentially life-threatening muscle weakness. Vyvgart is a human IgG1 antibody fragment that binds to FcRn, resulting in the reduction of circulating immunoglobulin G (IgG) autoantibodies. The action of IgG autoantibodies at the neuromuscular junction is a key driver of gMG.
Condition: Myasthenia Gravis
Type: drug