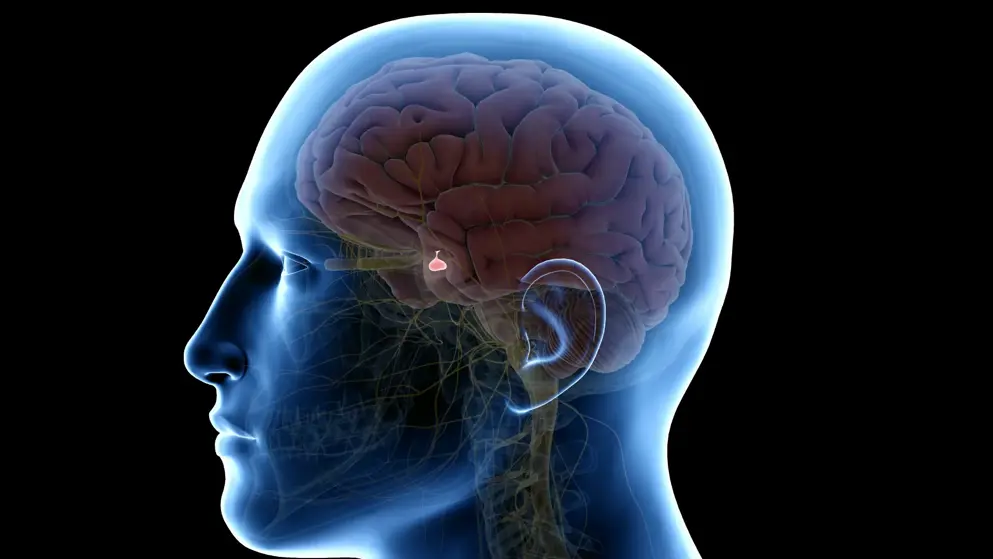
Ascendis Pharma A/S receives European approval for TransCon hGH for pediatric growth hormone deficiency.
TransCon hGH is a prodrug of somatropin that provides sustained release of unmodified somatropin (hGH) at predictable therapeutic levels in the body.
The EC approval is based on clinical results submitted in the Marketing Authorisation Application (MAA), including data from the Company’s Phase III heiGHt, fliGHt and enliGHten Trials, which collectively treated more than 300 pediatric patients diagnosed with GHD, as well as data from a non-clinical safety program.
In August 2021, the FDA approved TransCon hGH (as Skytrofa ) for the treatment of pediatric patients one year and older who weigh at least 11.5 kg and have growth failure due to inadequate secretion of endogenous growth hormone. TransCon hGH is also in development for pediatric GHD in Japan and China.