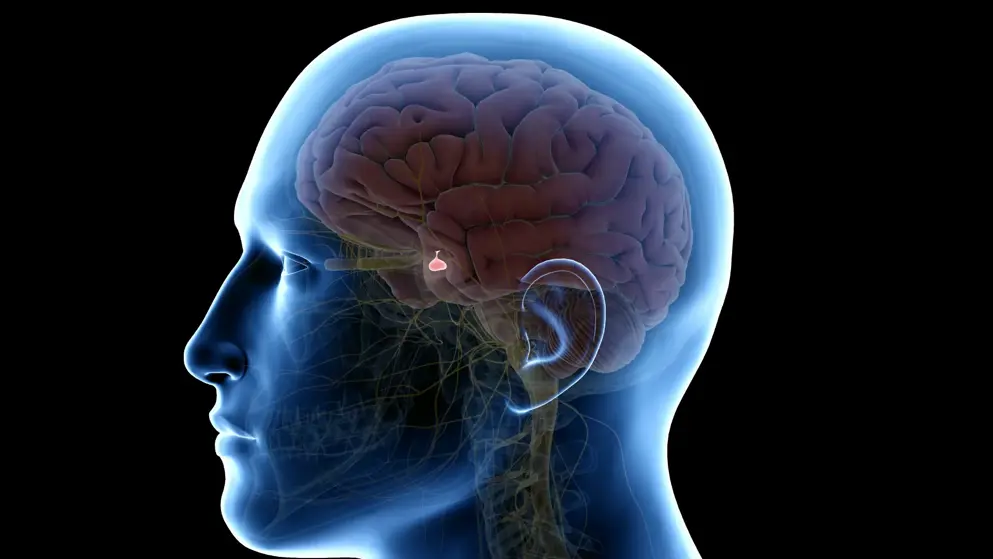
NDA submitted to PMDA in Japan for somatrogon, to treat pediatric patients with growth hormone deficiency.- Opko Health + Pfizer Japan.
OPKO Health announced that its partner, Pfizer Japan Inc., submitted a New Drug Application (NDA) to the Ministry of Health, Labour, and Welfare in Japan for somatrogon, a long-acting recombinant human growth hormone that is intended to be administered once-weekly for the treatment of pediatric patients with growth hormone deficiency (GHD). This submission is based on the results of the Japan Phase III and global Phase III clinical studies, conducted in subjects with pediatric GHD, in which the efficacy and safety of somatrogon administered once weekly were compared with Genotropin (somatropin), a recombinant human growth hormone for injection, administered once daily. In both studies, somatrogon showed comparable efficacy to Genotropin for the primary endpoint of annual height velocity at 12 months of treatment. Somatrogon was generally well tolerated in both studies, with comparable safety to that of Genotropin administered once-daily with respect to the types, numbers and severity of the adverse events observed between the treatment arms.