Phase III trial updates and a HER2-low debate
Review important developments in breast cancer with coverage of the 2022 San Antonio Breast Cancer Symposium (SABCS). From HER2-low to PARP inhibitors, debates, data presentations and more, get an overview of advances in breast cancer care across disease types.
- All about HER2: low, high, negative, and more with a lively debate about properly assessing this important biomarker
- PARP inhibitors, homologous recombination deficient tumours, mono- and combination therapies in multiple tumour types
- All the top highlights from this excellent breast cancer symposium
Watch the following video, in which Professor Coombes provides his view of some of the developments from SABCS 2022 relevant to clinical practice in the U.K.
Phase III trial updates
In the context of evolving therapies for HER2+ metastatic breast cancer (MBC), Dr Ian Krop presented primary results of the DESTINY-Breast02 phase III clinical trial1. Following patients on later line therapies post-trastuzumab emtansine (T-DM1), the study compared trastuzumab-deruxtecan (T-DXd, n = 406) to physician’s choice of therapy (TPC, n = 202). The primary endpoint of median progression-free survival (PFS) was 17.8 months with T-DXd compared to 6.9 months with TPC (hazard ratio [HR] 0.3589, 95% confidence interval [CI] 0.2840–0.4535). Median overall survival (OS) with T-DXd was 39.2 months, compared to 26.5 months with TPC (HR 0.6575, 95% CI 0.5023–0.8605). The median treatment duration was 11.3 months for T-DXd and approximately 4.5 months for TPC, with the most common treatment-emergent adverse events (TEAEs) being pneumonitis in 6.2% and interstitial lung disease in 3.2% of patients treated with T-DXd.
For a more direct comparison of treatments in MBC, Dr Sara A Hurvitz presented updated results on DESTINY-Breast032. This phase III trial followed 261 patients treated with T-DXd and 263 patients treated with T-DM1, all previously treated with trastuzumab and a taxane but facing recurrence within 6 months of therapy. Dr Hurvitz presented update data for the primary endpoint, a median PFS of 28.8 months with T-DXd and 6.8 months with T-DM1 (HR 0.33, 95% CI 0.26–0.43). The secondary endpoint, median OS, was not yet reached in either trial arm. The median treatment duration was 18.2 months for T-DXd and 6.9 months for T-DM1, with the most common TEAEs being nausea in (77.0%), vomiting (51.8%), and alopecia (39.7%) associated with T-DXd, and decreased platelet count (43.7%), increased aspartate aminotransferase (41.4%), and nausea (30.3%) associated with T-DM1. These data were simultaneously published in the Lancet3.
Figure 1. Median progression-free survival demonstrated in DESTINY-Breast 02 and DESTINY-Breast 03 (Adapted1,2).
In the next video, Professor Coombes summarises the evERA trial, a phase III study investigating giredestrant plus everolimus currently in progress.
A debate on HER2-low
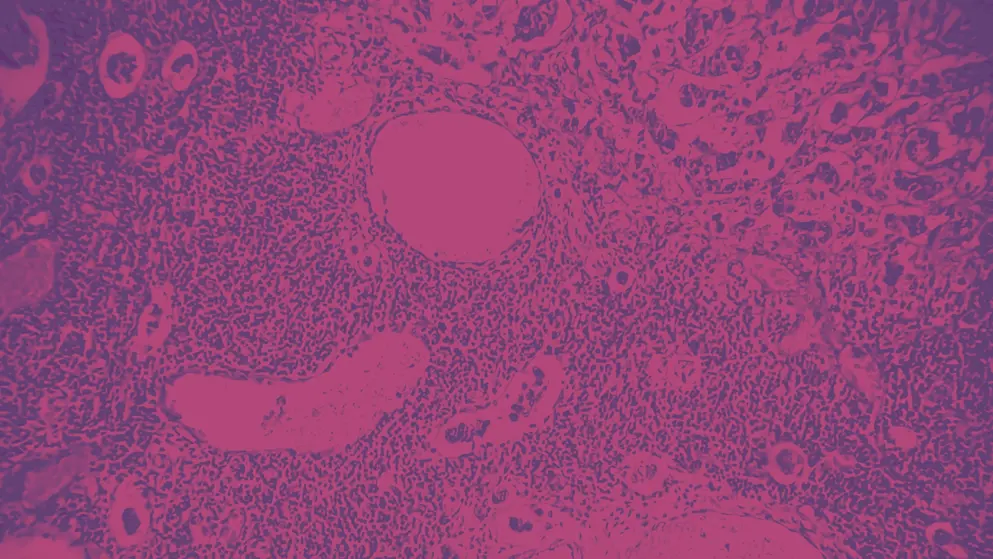
In addition to these data releases, a thought-provoking debate was held at SABCS on whether HER2-low is a separate entity or a biomarker. Current ASCO/CAP guidelines define HER2–negative (HER2–) tumours as immunohistochemical (IHC) staining IHC-0, IHC 1+, and 2+ (>10% cell stained) with paired negative in-situ hybridisation (ISH) assay4.
Figure 2. Classification of HER2 tumours according to immunohistochemical (IHC) staining (Adapted10).
Unmet need for high-sensitivity tumour characterisation
New developments in the management of HER2-low breast cancer (BC) first require assays with higher sensitivity for accurate identification. Pathologist Dr David Rimm discussed the challenges relating to pathology scoring for HER2-low tumours5. He noted that data presented by Dr Aleix Prat et al. on the Destiny-Breast04 trial found a 77% agreement between historical and central HER2-low status using IHC or ISH assays6; and that Dr Giuseppe Viale et al. revisited historical clinical and pathological data revealing that the prevalence of HER2-low among patients previously categorised as HER2– (n=789) was 67%7. Interestingly, the pathological agreement for HER2-low samples was higher than for HER2– samples. These HER2– findings were supported in soon to be published research by Robbins et al., who found that concordance rates of ten pathologists’ interpretation of IHC1+ versus not 1+ tumours was zero. Hence, patients who would potentially benefit from HER2-directed therapy first need an accurate assessment of their HER2 status.
Even pathologists point out that HER2 status is sometimes not easy to determine
Download an infographic overview of SABCS 2022 coverage
HER2-low is a separate entity
In historical data, metastasis-free survival shows poor outcomes with HER2-low (now categorised as HER2-zero) and HER2-high expression but better outcomes with normal expression (now categorised as HER2-low)8,9. Dr Guiseppe Curigliano proposed that if HER2-low is a separate entity, it must have a unique prognosis, a therapeutic clinical impact, or a unique biology with a robust assay to characterise this10.
Pooled data from four German neoadjuvant trials (n=3512) published by Denkert et al. showed that HER2-low was more common in HR+ (n=700) than in HR– (n=395) tumours11. HER2-low BCs generally had fewer grade III tumours and less proliferation (similar to data in the TALENT trial), with a significantly reduced pathological complete response (pCR) rate, particularly in the HR+ subgroup. Raghavendra et al. found that OS and disease-free survival (DFS) was significantly longer with HER2-low status than with HER2-zero status in patients with MBC12. The PenelopeB trial demonstrated therapeutic impact, where HR+/HER2 signalling shifted between ER and HER2-signalling following therapy. HER2-low status has shown an independent, negative prognostic value in patients with HR+/HER2- advanced BC treated with CDK4/6 inhibitor plus ET in the first-line setting13.
HER2-low tumours have unique biology and distinct molecular subtypes14. Gene expression also suggests that the drivers of resistance to neoadjuvant therapy (NeoAT) differ between HER2-low and HER2-zero tumours15.
HER2-low is not a separate entity
Dr Sara Tolaney argued HER2-low is not a separate entity as she considers it lacks unique clinical-pathological features and is likely driven by differences in ER expression16. As ER expression increases, HER2-low disease increases17.
Many studies have evaluated the prognostic role of HER2-low expression with no distinct difference in OS for HER2-low vs HER2-zero status. However, these studies are retrospective, heterogeneous, and lack central pathology review7,18. Pooled data from the previously mentioned German trials included ER low tumours within the HR+ cohort. As ER-low tumours were more likely to be HER2– and achieve pCR, this may explain why the HR+/HER2-low tumours had a lower pCR than HER2–. No significant difference in pCR was observed in any subgroup when corrected for HR expression.
In considering HER2 status, the interplay with ER or HR expression and other biomarkers cannot be ignored
Watch the following video to hear Professor Coombes share his SABCS 2022 highlights on biomarker developments in breast cancer management.
In the RxPONDER trial, chemoendocrine therapy (CET) improved DFS in premenopausal women, in both HER2-low and HER2– subgroups, but not for post-menopausal women. Thus, HER2 status should not be used to make therapeutic decisions in this population19.
In the following video, Professor Coombes discusses some of the developments in antibody-drug conjugates (ADCs) in breast cancer.
Fehrenbacher et al. found no benefit of adjuvant trastuzumab in HER2-low patients20, which contradicts results from the DESTINY-Breast04 study in HER2-low metastatic BC where T-Dxd doubled PFS compared to standard chemotherapy and improved OS irrespective of HR status or IHC level21. Thus, the activity of HER2-directed ADCs is not likely related to the blockade of an oncogenic driver but to the targeted delivery of a highly potent payload. HER2-low is therefore considered a biomarker for the benefit of ADCs targeting HER2. Many studies have confirmed the instability of HER2-low expression; this may be due to analytical factors, HER2 expression heterogeneity, or the biological evolution of the disease. Studies have also shown a shift in HER2-low cases from pre- to post-therapy, with discordance within the tissue from the same location, at the same time point, providing a dynamic definition of HER2-low status.
Novel quantitative HER2 assays are required to define HER2-low tumours precisely and how it adapts in response to therapy. Hence the HER2-low ‘entity’ debate continues and is likely to evolve.
 Professor Charles Coombes
Professor Charles Coombes
Watch the video below to meet the expert, Professor Charles Coombes.
Professor Coombes MD, PhD, FRCP, FMedSci heads the International Collaborative Cancer Trials Group and runs the Cancer Research UK Breast Cancer Group at Imperial College and Imperial College Healthcare NHS Trust. He has published more than 600 peer reviewed papers and has written 3 books on breast cancer. He established the CRUK Centre at Imperial College in 2008 and led the Department of Oncology at Imperial from 2000. His research has led to FDA and NICE approval of aromatase inhibitors for the treatment of breast cancer, and his studies on circulating cell-free DNA have led to this test being developed internationally. His research has led to the identification of several molecular targets in breast cancer, such as steroid sulfatase, histone deacetylases, fibroblast growth factor, CDK7 and CDK9, with treatments being developed subsequently.
of interest
are looking at
saved
next event
This content has been developed independently by Medthority who previously received educational funding from AstraZeneca in order to help provide its healthcare professional members with access to the highest quality medical and scientific information, education and associated relevant content.
