Dermatology
Medical conditions
Alopecia areata
Unmet needs and burden of disease
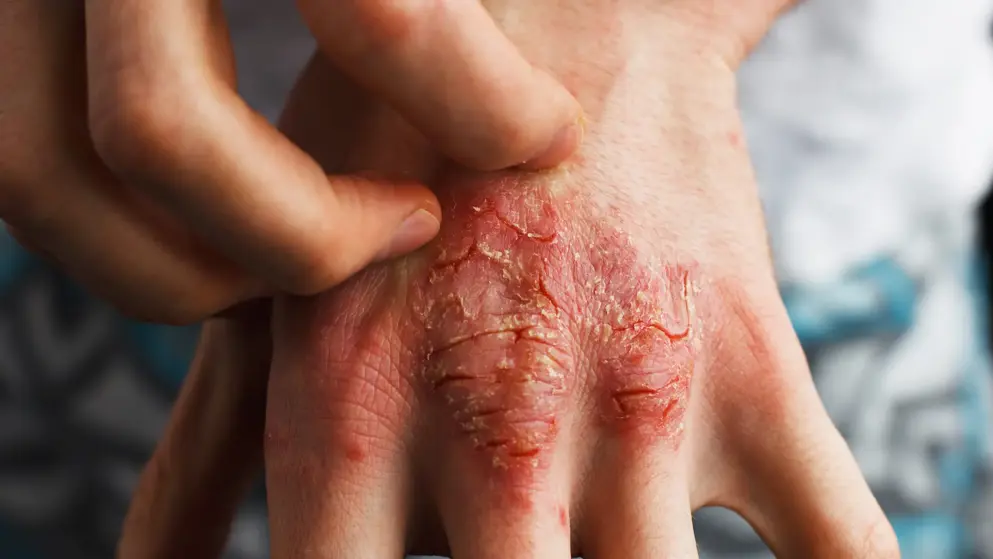
Dermatitis
Latest news, insights, and guideline updates
Epidermolysis bullosa
Disease information and guideline updates
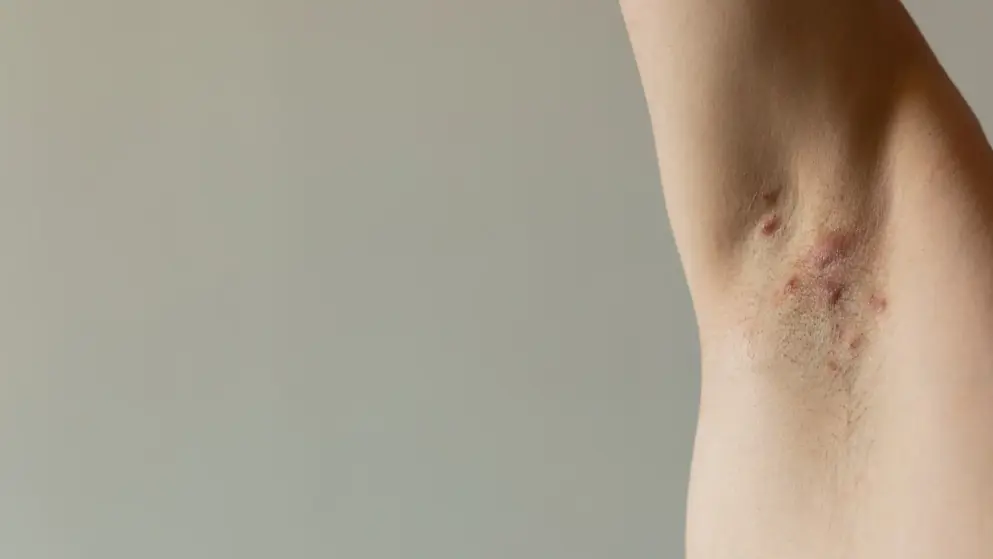
Hidradenitis suppurativa
Expert congress highlights on disease management
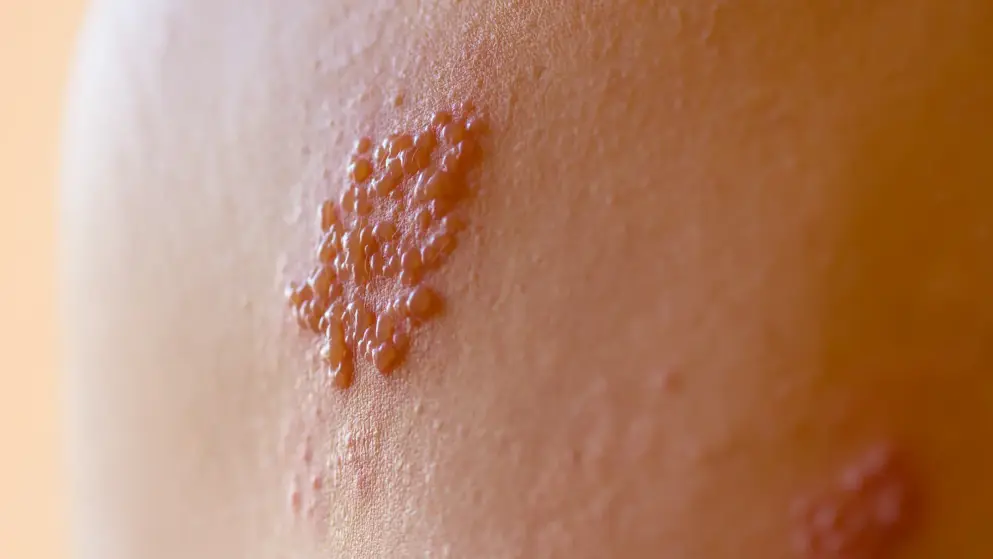
Herpes zoster
Test your knowledge with our quick quiz
Psoriasis
Real-world evidence and patient-centered care
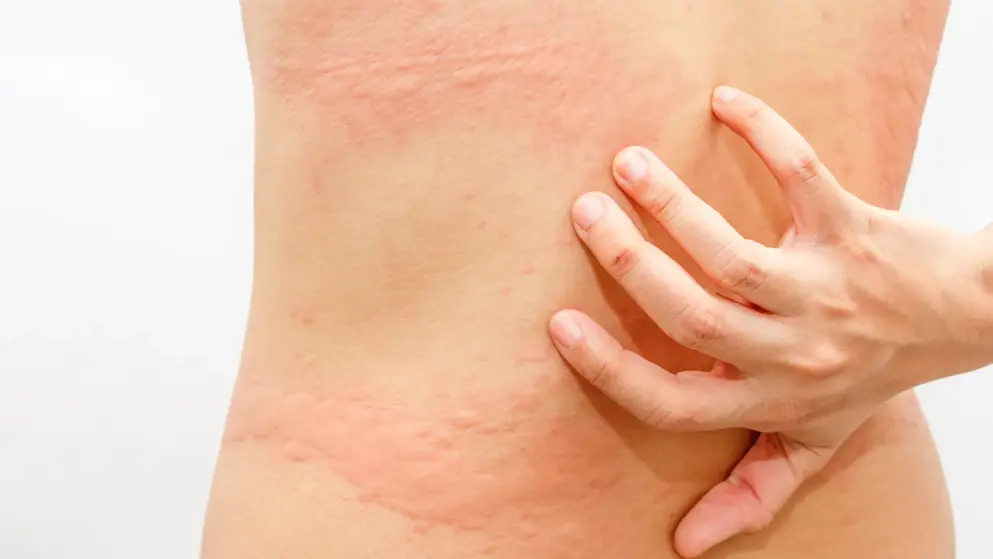
Urticaria
Latest updates and congress highlights
Latest resources
Alopecia areata: Progressing practice
Latest treatment options and patient insights
Expert insights on CSU management
The latest in CSU diagnosis and care
Hidradenitis Suppurativa Learning Zone
Explore updates on diagnosis, epidemiology, disease burden, pathophysiology, and future treatments for HS.
Advances in HS
EHSF 2024: Surgical updates and changes to practice
Alopecia areata webinar
Watch a webinar addressing alopecia areata in skin of color populations, chaired by Antonella Tosti.
Addressing inclusivity in AA assessment
View “Inclusive Care in Alopecia” for an in-depth look at how to better support skin of color populations with alopecia areata.
Roundtable: Biologics in plaque psoriasis
Watch expert roundtable on the use of biologics in moderate-to-severe plaque psoriasis, chaired by Professor Luis Puig.
Browse older resources
Atopic Dermatitis Learning Zone
Welcome to the Atopic Dermatitis Learning Zone, an educational resource, intended for healthcare professionals, that provides credible medical information on the epidemiology, pathophysiology and burden of atopic dermatitis, as well as diagnostic techniques, treatment regimens and guideline recommendations.
Improving Treatment Options for Childhood Psoriasis
This Learning Zone introduces you to our young patient Mia who, along with our paediatric psoriasis experts, helps us to describe the burden of childhood psoriasis for patients and their families, particularly the difficulties around diagnosis, comorbidities and limited treatment options.
Psoriasis Academy
When it comes to psoriasis, a knowledgeable healthcare professional is highly valued by patients (Pariser et al., 2016). With this in mind, we created The Psoriasis Academy, an online hub featuring succinct and easily accessible information for all healthcare professionals who want to know more about psoriasis and keep up to date with the latest developments.
Psoriasis Learning Zone
Psoriasis is a chronic inflammatory disease that often presents with disfiguring, scaling and erythematous plaques of the skin.
Herpes zoster quiz
What are the symptoms, tests, preventative measures and treatments for herpes zoster, more commonly known as shingles? How well do you know this common condition in the elderly? Find out by taking this quiz and learn more with additional content on Medthority.
Childhood psoriasis quiz
What impact does psoriasis have on children’s well-being and how should this condition be managed? Find out with this quiz and learn more about this important topic on Medthority.
of interest
are looking at
saved
next event