Psoriasis
Psoriasis is a chronic, immune-mediated, inflammatory skin disorder driven by genetic susceptibility and environmental triggers. Its pathogenesis involves dysregulated interactions between keratinocytes, dendritic cells, T cells, and other skin-resident immune components. Globally, psoriasis affects approximately 125 million individuals, representing a significant burden on public health.
Who is most at risk?
Psoriasis affects both sexes, though earlier onset is more frequently observed in females and those with a family history. The age of onset typically follows a bimodal distribution: 30–39 and 60–69 years in men, with onset occurring roughly a decade earlier in women.
What are the common symptoms of psoriasis?
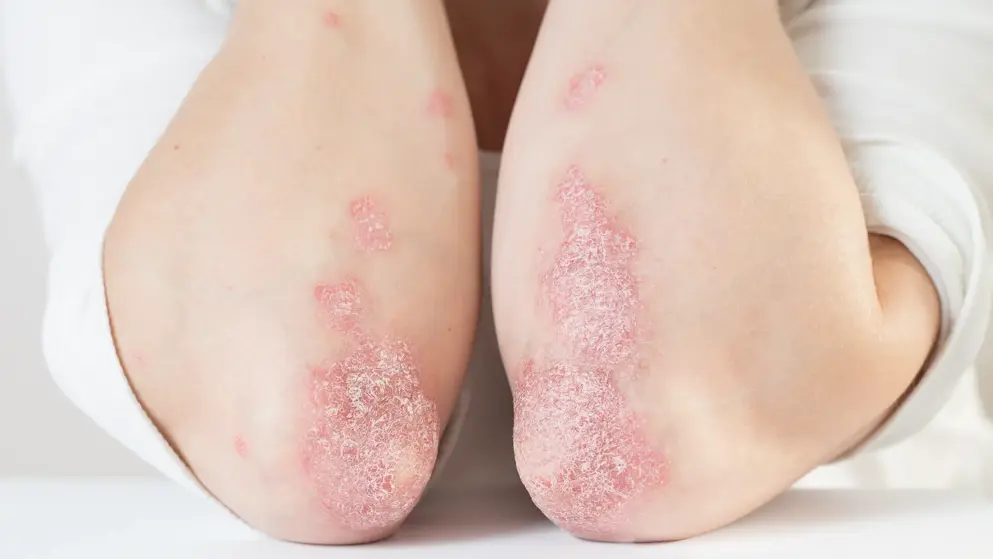
Plaque psoriasis, the most prevalent form, presents as well-demarcated, erythematous plaques with overlying silvery-white scale. Lesions commonly appear on the scalp, elbows, and knees, and are often pruritic or painful. Symptom severity and distribution vary by subtype and disease extent.
How does psoriasis impact quality of life?
Psoriasis can massively impair daily functioning, including occupational and sexual functioning. Many people report psychological distress, with embarrassment and social withdrawal frequently cited as major challenges.
What treatment options are currently available?
Management strategies depend on disease severity, prior treatment response, and patient preference. Therapeutic options include topical therapy, phototherapy, and systemic treatments, ranging from conventional immunosuppressants to targeted biologics. The primary treatment goal is to reduce epidermal hyperproliferation and inflammation while minimizing adverse effects.
Developed by EPG Health for Medthority, independently of any sponsor.
Browse older resources
Improving Treatment Options for Childhood Psoriasis
This Learning Zone introduces you to our young patient Mia who, along with our paediatric psoriasis experts, helps us to describe the burden of childhood psoriasis for patients and their families, particularly the difficulties around diagnosis, comorbidities and limited treatment options.
Psoriasis Academy
When it comes to psoriasis, a knowledgeable healthcare professional is highly valued by patients (Pariser et al., 2016). With this in mind, we created The Psoriasis Academy, an online hub featuring succinct and easily accessible information for all healthcare professionals who want to know more about psoriasis and keep up to date with the latest developments.
Psoriasis Learning Zone
Psoriasis is a chronic inflammatory disease that often presents with disfiguring, scaling and erythematous plaques of the skin.
Biologics in plaque psoriasis
Plaque psoriasis roundtable covering real-world evidence update, choosing the right treatment for the right patient and optimising response to biologics.
Related news and insights
Related guidelines
Childhood psoriasis quiz
What impact does psoriasis have on children’s well-being and how should this condition be managed? Find out with this quiz and learn more about this important topic on Medthority.