Duobrii
Summary of product characteristics
Adverse Reactions
6 ADVERSE REACTIONS • The most common adverse reactions are contact dermatitis (7%), application site pain (3%), folliculitis (2%), skin atrophy (2%), and excoriation (2%). ( 6.1 ) To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. 6.1 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In randomized, double-blind, multicenter, vehicle-controlled clinical trials, 410 adults with plaque psoriasis were treated with DUOBRII Lotion or vehicle lotion and had post-baseline safety data. Subjects applied DUOBRII Lotion or vehicle lotion once daily for up to 8 weeks. Table 1 presents adverse reactions that occurred in at least 1% of subjects treated with DUOBRII Lotion and more frequently than in vehicle-treated subjects. Table 1: Adverse Reactions Occurring in ≥1% of the Subjects Treated with DUOBRII Lotion through Week 8 Adverse Reaction DUOBRII Lotion (N=270) Vehicle Lotion (N=140) Contact Dermatitis 20 (7%) 0 Application Site Pain 7 (3%) 1 (1%) Folliculitis 5 (2%) 0 Skin Atrophy 5 (2%) 0 Excoriation 5 (2%) 0 Rash 4 (1%) 0 Skin Abrasion 3 (1%) 0 Skin Exfoliation 2 (1%) 0
Contraindications
4 CONTRAINDICATIONS DUOBRII Lotion is contraindicated in pregnancy ( 4.1 , 8.1 ) 4.1 Pregnancy DUOBRII Lotion is contraindicated in pregnancy [ see Warnings and Precautions (5.1) , Use in Specific Populations ( 8.1 , 8.3) ].
Description
11 DESCRIPTION DUOBRII Lotion is a combination product with halobetasol propionate and tazarotene as the active ingredients in a white to off-white lotion formulation intended for topical use. Halobetasol propionate is a synthetic corticosteroid. The chemical name for halobetasol propionate is [(6S,9R,16S,17R)-17-(2-chloroacetyl)-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propanoate. The structural formula for halobetasol propionate is represented below: Molecular Formula: C 25 H 31 ClF 2 O 5 Molecular Weight: 484.96 Tazarotene is a member of the acetylenic class of retinoids. The chemical name for tazarotene is 6-[(3,4-Dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl]-3-pyridinecarboxylic acid ethyl ester. The structural formula for tazarotene is represented below: Molecular Formula: C 21 H 21 NO 2 S Molecular Weight: 351.46 Each gram of DUOBRII Lotion contains 0.1 mg (0.01%) halobetasol propionate and 0.45 mg (0.045%) tazarotene in a white to off-white lotion base consisting of carbomer copolymer type B, carbomer homopolymer type A, diethyl sebacate, edetate disodium dihydrate, light mineral oil, methylparaben, propylparaben, purified water, sodium hydroxide, sorbitan monooleate and sorbitol solution, 70%. chem1.jpg chem2.jpg
Dosage And Administration
2 DOSAGE AND ADMINISTRATION Apply a thin layer of DUOBRII Lotion once daily to cover only affected areas and rub in gently. If a bath or shower is taken prior to application, the skin should be dry before applying the lotion. The total dosage should not exceed approximately 50 g per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis [see Warnings and Precautions (5.2) ] . Do not use with occlusive dressings unless directed by a physician. Discontinue treatment when control is achieved. Avoid application of DUOBRII Lotion on the face, groin, or in the axillae. DUOBRII Lotion is not for oral, ophthalmic, or intravaginal use. • Apply a thin layer of DUOBRII Lotion to the affected areas once daily. ( 2 ) • Not for oral, ophthalmic, or intravaginal use. ( 2 )
Indications And Usage
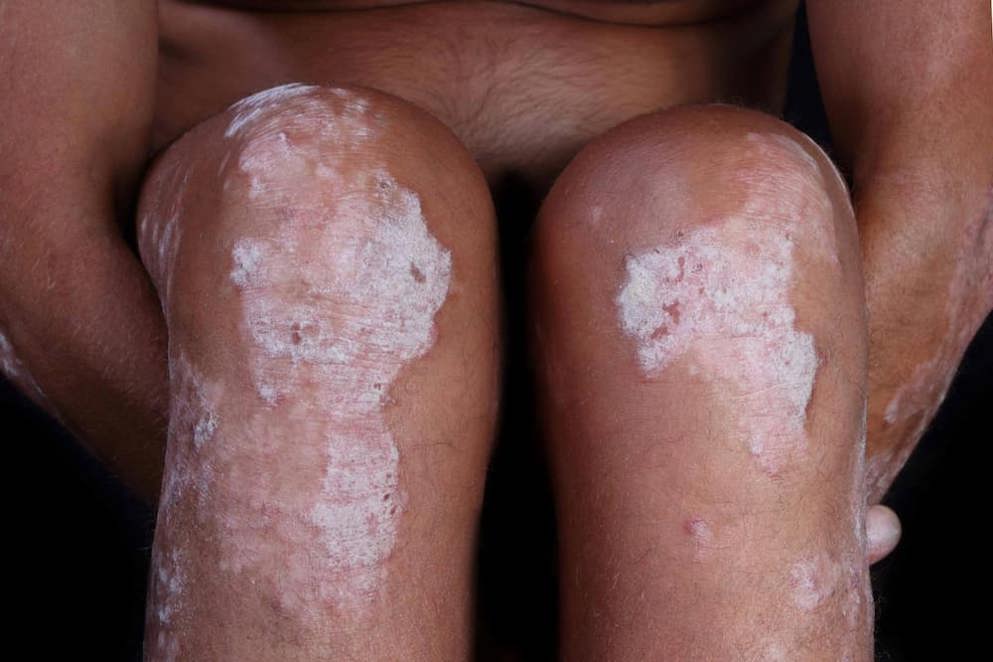
1 INDICATIONS AND USAGE DUOBRII ® (halobetasol propionate and tazarotene) Lotion, 0.01%/0.045% is indicated for the topical treatment of plaque psoriasis in adults. DUOBRII Lotion is a combination of halobetasol propionate and tazarotene indicated for the topical treatment of plaque psoriasis in adults. ( 1 )
Adverse Reactions Table
Adverse Reaction | DUOBRII Lotion (N=270) | Vehicle Lotion (N=140) |
Contact Dermatitis | 20 (7%) | 0 |
Application Site Pain | 7 (3%) | 1 (1%) |
Folliculitis | 5 (2%) | 0 |
Skin Atrophy | 5 (2%) | 0 |
Excoriation | 5 (2%) | 0 |
Rash | 4 (1%) | 0 |
Skin Abrasion | 3 (1%) | 0 |
Skin Exfoliation | 2 (1%) | 0 |
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action in plaque psoriasis is unknown. Tazarotene is a retinoid prodrug which is converted to its active form, tazarotenic acid, the carboxylic acid of tazarotene, by deesterification. Tazarotenic acid binds to all three members of the retinoic acid receptor (RAR) family: RARα, RARβ and RARγ, but shows relative selectivity for RARβ, and RARγ and may modify gene expression. The clinical significance of these findings for the treatment of plaque psoriasis is unknown. 12.2 Pharmacodynamics A vasoconstrictor assay in healthy subjects with DUOBRII Lotion indicated that it is in the high to super-high range of potency as compared to other topical corticosteroids; however, similar blanching scores do not necessarily imply therapeutic equivalence. The potential for hypothalamic-pituitary-adrenal (HPA) axis suppression was evaluated in a study in adult subjects with moderate to severe plaque psoriasis. A median dose of 8.2 grams DUOBRII Lotion was applied once daily for 8 weeks and 20 subjects were assessed for HPA axis suppression at Weeks 4 and 8. HPA axis suppression was observed in 3 out of 20 (15%) subjects at Week 4. None of the 20 (0%) subjects had HPA axis suppression at Week 8. In this study, the criteria for HPA axis suppression was a serum cortisol level of less than or equal to 18 micrograms per deciliter 30 minutes after stimulation with cosyntropin (adrenocorticotropic hormone). [See Warnings and Precautions (5.2) .] The pharmacodynamics of tazarotene is unknown. 12.3 Pharmacokinetics Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. Systemic exposure following topical application of DUOBRII Lotion was evaluated in the same study that evaluated the HPA axis suppression. It was an open-label, randomized, pharmacokinetics (PK) study conducted in subjects aged 18 years and older with moderate to severe plaque psoriasis affecting at least 20% body surface area. The PK of halobetasol propionate, tazarotene, and tazarotenic acid was evaluated in 22 subjects following application of DUOBRII Lotion to the affected area once daily for 28 days. Systemic concentrations of halobetasol propionate (lower limit of quantification (LLOQ) = 50 pg/mL) and tazarotene (LLOQ = 5 pg/mL) on Day 28 were quantifiable in 13 and 18 out of a total number of 22 subjects, respectively. Tazarotenic acid (LLOQ = 5 pg/mL) was quantifiable in all subjects. Systemic exposure of the three moieties was at or near steady state by Day 28. The mean (standard deviation) of PK parameters on Day 28 is shown in Table 2. Table 2:PK Parameters of Halobetasol Propionate, Tazarotene, and Tazarotenic Acid Following Once Daily Administration of DUOBRII Lotion for 28 Days in Subjects with Moderate to Severe Plaque Psoriasis Mean (Standard Deviation) (N=22) PK Parameters Halobetasol Propionate Tazarotene Tazarotenic Acid Day 28 C max (pg/mL) 101.9 (135.4) 24.6 (27.3) 523.4 (523.3) AUC 0-24 (pg*hr/mL) 1300 (1959) 273 (403) 9954 (10091)
Clinical Pharmacology Table
Mean (Standard Deviation) (N=22) | ||||
PK Parameters | Halobetasol Propionate | Tazarotene | Tazarotenic Acid | |
Day 28 | Cmax (pg/mL) | 101.9 (135.4) | 24.6 (27.3) | 523.4 (523.3) |
AUC0-24 (pg*hr/mL) | 1300 (1959) | 273 (403) | 9954 (10091) | |
Mechanism Of Action
12.1 Mechanism of Action Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action in plaque psoriasis is unknown. Tazarotene is a retinoid prodrug which is converted to its active form, tazarotenic acid, the carboxylic acid of tazarotene, by deesterification. Tazarotenic acid binds to all three members of the retinoic acid receptor (RAR) family: RARα, RARβ and RARγ, but shows relative selectivity for RARβ, and RARγ and may modify gene expression. The clinical significance of these findings for the treatment of plaque psoriasis is unknown.
Pharmacodynamics
12.2 Pharmacodynamics A vasoconstrictor assay in healthy subjects with DUOBRII Lotion indicated that it is in the high to super-high range of potency as compared to other topical corticosteroids; however, similar blanching scores do not necessarily imply therapeutic equivalence. The potential for hypothalamic-pituitary-adrenal (HPA) axis suppression was evaluated in a study in adult subjects with moderate to severe plaque psoriasis. A median dose of 8.2 grams DUOBRII Lotion was applied once daily for 8 weeks and 20 subjects were assessed for HPA axis suppression at Weeks 4 and 8. HPA axis suppression was observed in 3 out of 20 (15%) subjects at Week 4. None of the 20 (0%) subjects had HPA axis suppression at Week 8. In this study, the criteria for HPA axis suppression was a serum cortisol level of less than or equal to 18 micrograms per deciliter 30 minutes after stimulation with cosyntropin (adrenocorticotropic hormone). [See Warnings and Precautions (5.2) .] The pharmacodynamics of tazarotene is unknown.
Pharmacokinetics
12.3 Pharmacokinetics Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. Systemic exposure following topical application of DUOBRII Lotion was evaluated in the same study that evaluated the HPA axis suppression. It was an open-label, randomized, pharmacokinetics (PK) study conducted in subjects aged 18 years and older with moderate to severe plaque psoriasis affecting at least 20% body surface area. The PK of halobetasol propionate, tazarotene, and tazarotenic acid was evaluated in 22 subjects following application of DUOBRII Lotion to the affected area once daily for 28 days. Systemic concentrations of halobetasol propionate (lower limit of quantification (LLOQ) = 50 pg/mL) and tazarotene (LLOQ = 5 pg/mL) on Day 28 were quantifiable in 13 and 18 out of a total number of 22 subjects, respectively. Tazarotenic acid (LLOQ = 5 pg/mL) was quantifiable in all subjects. Systemic exposure of the three moieties was at or near steady state by Day 28. The mean (standard deviation) of PK parameters on Day 28 is shown in Table 2. Table 2:PK Parameters of Halobetasol Propionate, Tazarotene, and Tazarotenic Acid Following Once Daily Administration of DUOBRII Lotion for 28 Days in Subjects with Moderate to Severe Plaque Psoriasis Mean (Standard Deviation) (N=22) PK Parameters Halobetasol Propionate Tazarotene Tazarotenic Acid Day 28 C max (pg/mL) 101.9 (135.4) 24.6 (27.3) 523.4 (523.3) AUC 0-24 (pg*hr/mL) 1300 (1959) 273 (403) 9954 (10091)
Pharmacokinetics Table
Mean (Standard Deviation) (N=22) | ||||
PK Parameters | Halobetasol Propionate | Tazarotene | Tazarotenic Acid | |
Day 28 | Cmax (pg/mL) | 101.9 (135.4) | 24.6 (27.3) | 523.4 (523.3) |
AUC0-24 (pg*hr/mL) | 1300 (1959) | 273 (403) | 9954 (10091) | |
Effective Time
20200101
Version
6
Dosage Forms And Strengths
3 DOSAGE FORMS AND STRENGTHS Lotion, 0.01%/0.045% Each gram of DUOBRII Lotion contains 0.1 mg (0.01%) halobetasol propionate and 0.45 mg (0.045%) tazarotene in a white to off-white lotion. Lotion, 0.01%/0.045% ( 3 ) Each gram of DUOBRII Lotion contains 0.1 mg (0.01%) halobetasol propionate and 0.45 mg (0.045%) tazarotene. ( 3 )
Spl Product Data Elements
Duobrii halobetasol propionate and tazarotene HALOBETASOL PROPIONATE HALOBETASOL TAZAROTENE TAZAROTENE CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) DIETHYL SEBACATE EDETATE DISODIUM LIGHT MINERAL OIL METHYLPARABEN PROPYLPARABEN WATER SODIUM HYDROXIDE SORBITAN MONOOLEATE SORBITOL
Carcinogenesis And Mutagenesis And Impairment Of Fertility
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility Long-term animal studies have not been performed to evaluate the carcinogenic potential of halobetasol propionate. A long-term study of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no indications of increased carcinogenic risks. Based on pharmacokinetic data from a shorter term study in rats, the highest dose of 0.125 mg/kg/day was anticipated to give systemic exposure in the rat equivalent to 1.4 times the MRHD (based on AUC comparison). A long-term study with topical application of up to 0.1% of tazarotene in a gel formulation in mice terminated at 88 weeks showed that dose levels of 0.05, 0.125, 0.25, and 1 mg/kg/day (reduced to 0.5 mg/kg/day for males after 41 weeks due to severe dermal irritation) revealed no apparent carcinogenic effects when compared to vehicle control animals. Tazarotenic acid systemic exposures at the highest dose was 35 times the MRHD (based on AUC comparison). Halobetasol propionate was not genotoxic in the Ames assay, in the sister chromatid exchange test in Chinese hamster somatic cells, in chromosome aberration studies of germinal and somatic cells of rodents, and in a mammalian spot test. Positive mutagenicity effects were observed in a mouse lymphoma gene mutation assay in vitro and in a Chinese hamster micronucleus test. Tazarotene was non-mutagenic in the Ames assay and did not produce structural chromosomal aberrations in human lymphocytes. Tazarotene was non-mutagenic in the CHO/HGPRT mammalian cell forward gene mutation assay and was non-clastogenic in an in vivo mouse micronucleus test. Studies in rats following oral administration of halobetasol propionate at dose levels up to 0.05 mg/kg/day, approximately 0.53 times the MRHD based on BSA comparisons, indicated no impairment of fertility or general reproductive performance. No impairment of fertility occurred in rats when male animals were treated for 70 days prior to mating and female animals were treated for 14 days prior to mating and continuing through gestation and lactation with topical doses of a tazarotene gel formulation up to 0.125 mg/kg/day. Based on data from another study, the systemic drug exposure in the rat at the highest dose was 5 times the MRHD (based on AUC comparison). No impairment of mating performance or fertility was observed in male rats treated for 70 days prior to mating with oral doses of up to 1 mg/kg/day tazarotene, which produced a systemic exposure 17 times the MRHD (based on AUC comparison). No impairment of mating performance or fertility was observed in female rats treated for 15 days prior to mating and continuing through gestation day 7 with oral doses of tazarotene up to 2 mg/kg/day. However, there was a significant decrease in the number of estrous stages and an increase in developmental effects at that dose, which produced a systemic exposure 30 times the MRHD (based on AUC comparison).
Nonclinical Toxicology
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility Long-term animal studies have not been performed to evaluate the carcinogenic potential of halobetasol propionate. A long-term study of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no indications of increased carcinogenic risks. Based on pharmacokinetic data from a shorter term study in rats, the highest dose of 0.125 mg/kg/day was anticipated to give systemic exposure in the rat equivalent to 1.4 times the MRHD (based on AUC comparison). A long-term study with topical application of up to 0.1% of tazarotene in a gel formulation in mice terminated at 88 weeks showed that dose levels of 0.05, 0.125, 0.25, and 1 mg/kg/day (reduced to 0.5 mg/kg/day for males after 41 weeks due to severe dermal irritation) revealed no apparent carcinogenic effects when compared to vehicle control animals. Tazarotenic acid systemic exposures at the highest dose was 35 times the MRHD (based on AUC comparison). Halobetasol propionate was not genotoxic in the Ames assay, in the sister chromatid exchange test in Chinese hamster somatic cells, in chromosome aberration studies of germinal and somatic cells of rodents, and in a mammalian spot test. Positive mutagenicity effects were observed in a mouse lymphoma gene mutation assay in vitro and in a Chinese hamster micronucleus test. Tazarotene was non-mutagenic in the Ames assay and did not produce structural chromosomal aberrations in human lymphocytes. Tazarotene was non-mutagenic in the CHO/HGPRT mammalian cell forward gene mutation assay and was non-clastogenic in an in vivo mouse micronucleus test. Studies in rats following oral administration of halobetasol propionate at dose levels up to 0.05 mg/kg/day, approximately 0.53 times the MRHD based on BSA comparisons, indicated no impairment of fertility or general reproductive performance. No impairment of fertility occurred in rats when male animals were treated for 70 days prior to mating and female animals were treated for 14 days prior to mating and continuing through gestation and lactation with topical doses of a tazarotene gel formulation up to 0.125 mg/kg/day. Based on data from another study, the systemic drug exposure in the rat at the highest dose was 5 times the MRHD (based on AUC comparison). No impairment of mating performance or fertility was observed in male rats treated for 70 days prior to mating with oral doses of up to 1 mg/kg/day tazarotene, which produced a systemic exposure 17 times the MRHD (based on AUC comparison). No impairment of mating performance or fertility was observed in female rats treated for 15 days prior to mating and continuing through gestation day 7 with oral doses of tazarotene up to 2 mg/kg/day. However, there was a significant decrease in the number of estrous stages and an increase in developmental effects at that dose, which produced a systemic exposure 30 times the MRHD (based on AUC comparison).
Application Number
NDA209354
Brand Name
Duobrii
Generic Name
halobetasol propionate and tazarotene
Product Ndc
0187-0653
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Package Label Principal Display Panel
Package/Label Display Panel NDC 0187-0653-01 Duobrii ® (halobetasol propionate and tazarotene) Lotion 0.01%/ 0.045% Rx only Net Wt. 100g For Topical Use Only Not for Eye Use Ortho Dermatologics carton.jpg
Information For Patients
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling ( Patient Information ). This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all administration instructions or all possible adverse or unintended effects. Advise patients using DUOBRII Lotion of the following information and instructions: Important Administration Instructions If undue irritation (redness, peeling, or discomfort) occurs, reduce frequency of application or temporarily interrupt treatment. Treatment may be resumed once irritation subsides [ see Dosage and Administration ( 2 ) and Warnings and Precautions ( 5.2 ) ]. Inform patients that total dosage should not exceed 50 grams per week [see Dosage and Administration (2) ] . Instruct patients to avoid bandaging, wrapping or otherwise occluding the treatment area(s), unless directed by physician. Advise patients to avoid use on the face, groin, or axillae [see Dosage and Administration ( 2 )] . Inform patients that DUOBRII Lotion is for external use only. Advise patients that DUOBRII Lotion is not for oral, ophthalmic, or intravaginal use [see Dosage and Administration ( 2 )]. Fetal risk is associated with DUOBRII Lotion for females of reproductive potential. Advise patients to use an effective method of contraception during treatment to avoid pregnancy. Advise the patient to stop medication if she becomes pregnant and call her doctor [ see Contraindications ( 4.1 ), Warnings and Precautions ( 5.1 ) and Use in Specific Populations ( 8.1 ) ]. Breastfeeding women should not apply DUOBRII Lotion directly to the nipple and areola to avoid directly exposing the infant [see Use in Specific Populations ( 8.2 )] . Avoid exposure of the treated areas to either natural or artificial sunlight, including tanning beds and sunlamps. Use sunscreen and protective clothing if exposure to sunlight is unavoidable when using DUOBRII Lotion [see Warnings and Precautions ( 5.4 )] . HPA Axis Suppression and Other Unwanted Systemic Glucocorticoid Effects DUOBRII Lotion may cause HPA axis suppression. Advise patients that use of topical corticosteroids, including DUOBRII Lotion, may require periodic evaluation for HPA axis suppression. Topical corticosteroids may have other endocrine effects. Concomitant use of multiple corticosteroid-containing products may increase the total systemic exposure to topical corticosteroids [see Warnings and Precautions (5.2) ] . Local Adverse Reactions Inform patients that DUOBRII Lotion may cause local adverse reactions. These reactions may be more likely to occur with occlusive use or use of DUOBRII Lotion. If undue irritation (redness, peeling, or discomfort) occurs, reduce frequency of application or temporarily interrupt treatment. Treatment may be resumed once irritation subsides, unless allergic contact dermatitis is identified [see Warnings and Precautions (5.3) ]. Ophthalmic Adverse Reactions Advise patients to report any visual symptoms to their healthcare providers. Distributed by: Bausch Health US, LLC Bridgewater, NJ 08807 USA Manufactured by: Bausch Health Companies Inc. Laval, Quebec H7L 4A8, Canada U.S. Patent Numbers: 6,517,847; 8,809,307; 10,251,895 and 10,478,502 DUOBRII is a trademark of Bausch Health Companies Inc. or its affiliates. © 2020 Bausch Health Companies Inc. or its affiliates 9645602
Clinical Studies
14 CLINICAL STUDIES The safety and efficacy of once daily use of DUOBRII Lotion for the treatment of moderate to severe plaque psoriasis were assessed in two prospective, multicenter, randomized, double-blind clinical trials (Trial 1 [NCT02462070] and Trial 2 [NCT02462122]). These trials were conducted in 418 subjects 18 years of age and older with moderate to severe plaque psoriasis that covered a body surface area (BSA) between 3% and 12% excluding the face, scalp, palms, soles, axillae, and intertriginous areas. Disease severity was determined by a 5-grade Investigator’s Global Assessment (IGA). Subjects applied DUOBRII Lotion or vehicle to all affected areas once daily for up to 8 weeks. All subjects returned for a 4-week follow-up visit (Week 12 visit) where safety and efficacy were evaluated. The primary efficacy endpoint was the proportion of subjects with “treatment success” at Week 8. Treatment success was defined as at least a 2-grade improvement from baseline in the IGA score and an IGA score equating to “clear” or “almost clear”. Table 3 lists the primary efficacy results for Trials 1 and 2. The secondary efficacy endpoints evaluated treatment success sequentially at Weeks 12, 6, 4, and 2. Figure 1 shows the primary and secondary efficacy results over time. Table 3: Primary Efficacy Outcomes in Subjects with Moderate to Severe Plaque Psoriasis at Week 8 Trial 1 Trial 2 DUOBRII Vehicle DUOBRII Vehicle N=135 N=68 N=141 N=74 IGA Treatment Success at Week 8 Treatment success was defined as at least a 2-grade improvement from baseline in IGA score and an IGA score equating to “clear” or “almost clear”. Clear = no evidence of scaling, no evidence of erythema, no evidence of plaque elevation above normal skin level. Almost clear = some plaques with fine scales, faint pink/light red erythema on most plaques, slight or barely perceptible elevation of plaques above normal skin level. 36% 7% 45% 13% Figure 1 : Efficacy Results* over Time * The treatment difference at Week 2 in Trial 1 was not statistically significant. image-01.jpg
Clinical Studies Table
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| DUOBRII | Vehicle | DUOBRII | Vehicle | |
| N=135 | N=68 | N=141 | N=74 | |
IGA Treatment Success at Week 8 | 36% | 7% | 45% | 13% |
Geriatric Use
8.5 Geriatric Use Of the 270 subjects exposed to DUOBRII Lotion in clinical trials, 39 subjects were 65 years or older. Clinical trials of DUOBRII Lotion did not include sufficient numbers of subjects age 65 years and older to determine whether they respond differently from younger subjects.
Pediatric Use
8.4 Pediatric Use Safety and effectiveness of DUOBRII Lotion in pediatric patients under the age of 18 years have not been evaluated. Because of higher skin-surface-area-to-body-mass ratios, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during or after withdrawal of treatment. Adverse reactions including striae have been reported with use of topical corticosteroids in infants and children [see Warnings and Precautions (5.2) ] . HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema [see Warnings and Precautions (5.2) ] .
Pregnancy
8.1 Pregnancy Risk Summary Based on data from animal reproduction studies, retinoid pharmacology, and the potential for systemic absorption, DUOBRII Lotion may cause fetal harm when administered to a pregnant female and is contraindicated during pregnancy. Safety in pregnant females has not been established. The potential risk to the fetus outweighs the potential benefit to the mother from DUOBRII Lotion during pregnancy; therefore, DUOBRII Lotion should be discontinued as soon as pregnancy is recognized [see Contraindications (4) , Warnings and Precautions (5.1) , Clinical Pharmacology (12.3) ] . Observational studies suggest an increased risk of low birthweight in infants with the maternal use of potent or very potent topical corticosteroids ( see Data ). In animal reproduction studies with pregnant rats, reduced fetal body weights and reduced skeletal ossification were observed after topical administration of a tazarotene gel formulation during the period of organogenesis at a dose 11 times the maximum recommended human dose (MRHD) (based on AUC comparison). In animal reproduction studies with pregnant rabbits, single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies were observed after topical administration of a tazarotene gel formulation at 116 times the MRHD (based on AUC comparison) (see Data) . In animal reproduction studies with pregnant rats and rabbits, malformations, fetal toxicity, developmental delays, and/or behavioral delays were observed after oral administration of tazarotene during the period of organogenesis at doses 9 and 228 times, respectively, the MRHD (based on AUC comparison). In pregnant rats, decreased litter size, decreased numbers of live fetuses, decreased fetal body weights, and increased malformations were observed after oral administration of tazarotene prior to mating through early gestation at doses 9 times the MRHD (based on AUC comparison) (see Data) . In animal reproduction studies, increased malformations, including cleft palate and omphalocele, were observed after oral administration of halobetasol propionate during the period of organogenesis to pregnant rats and rabbits (see Data) . The available data do not support relevant comparisons of systemic halobetasol propionate exposures achieved in the animal studies to exposures observed in humans after topical use of DUOBRII Lotion. The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies. Data Human Data Available observational studies in pregnant women did not identify a drug-associated risk of major birth defects, preterm delivery, or fetal mortality with the use of topical corticosteroids of any potency. However, when the dispensed amount of potent or very potent topical corticosteroids exceeded 300 g during the entire pregnancy, maternal use was associated with an increased risk of low birth weight in infants. Animal Data Halobetasol propionate has been shown to cause malformations in rats and rabbits when given orally during organogenesis at doses of 0.04 to 0.1 mg/kg/day in rats and 0.01 mg/kg/day in rabbits. Halobetasol propionate was embryotoxic in rabbits but not in rats. Cleft palate was observed in both rats and rabbits. Omphalocele was seen in rats but not in rabbits. In an embryofetal development study in rats, a tazarotene gel formulation, 0.5% (0.25 mg/kg/day tazarotene) was topically administered to pregnant rats during gestation days 6 through 17. Reduced fetal body weights and reduced skeletal ossification occurred at this dose (11 times the MRHD based on AUC comparison). In an embryofetal development study in rabbits, a tazarotene gel formulation (0.5%, 0.25 mg/kg/day tazarotene) was topically administered to pregnant rabbits during gestation days 6 through 18. Single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies were noted at this dose (116 times the MRHD based on AUC comparison). When tazarotene was given orally to animals, developmental delays were seen in rats; malformations and post-implantation loss were observed in rats and rabbits at doses producing 9 and 228 times, respectively, the MRHD (based on AUC comparisons). In female rats orally administered 2 mg/kg/day of tazarotene from 15 days before mating through gestation day 7, classic developmental effects of retinoids including decreased number of implantation sites, decreased litter size, decreased numbers of live fetuses, and decreased fetal body weights were observed at this dose (16 times the MRHD based on AUC comparison). A low incidence of retinoid-related malformations was observed at that dose. In a pre- and postnatal development toxicity study, topical administration of a tazarotene gel formulation (0.125 mg/kg/day) to pregnant female rats from gestation day 16 through lactation day 20 reduced pup survival but did not affect the reproductive capacity of the offspring. Based on data from another study, the systemic drug exposure in the rat at this dose would be equivalent to 5 times the MRHD (based on AUC comparison).
Use In Specific Populations
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Risk Summary Based on data from animal reproduction studies, retinoid pharmacology, and the potential for systemic absorption, DUOBRII Lotion may cause fetal harm when administered to a pregnant female and is contraindicated during pregnancy. Safety in pregnant females has not been established. The potential risk to the fetus outweighs the potential benefit to the mother from DUOBRII Lotion during pregnancy; therefore, DUOBRII Lotion should be discontinued as soon as pregnancy is recognized [see Contraindications (4) , Warnings and Precautions (5.1) , Clinical Pharmacology (12.3) ] . Observational studies suggest an increased risk of low birthweight in infants with the maternal use of potent or very potent topical corticosteroids ( see Data ). In animal reproduction studies with pregnant rats, reduced fetal body weights and reduced skeletal ossification were observed after topical administration of a tazarotene gel formulation during the period of organogenesis at a dose 11 times the maximum recommended human dose (MRHD) (based on AUC comparison). In animal reproduction studies with pregnant rabbits, single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies were observed after topical administration of a tazarotene gel formulation at 116 times the MRHD (based on AUC comparison) (see Data) . In animal reproduction studies with pregnant rats and rabbits, malformations, fetal toxicity, developmental delays, and/or behavioral delays were observed after oral administration of tazarotene during the period of organogenesis at doses 9 and 228 times, respectively, the MRHD (based on AUC comparison). In pregnant rats, decreased litter size, decreased numbers of live fetuses, decreased fetal body weights, and increased malformations were observed after oral administration of tazarotene prior to mating through early gestation at doses 9 times the MRHD (based on AUC comparison) (see Data) . In animal reproduction studies, increased malformations, including cleft palate and omphalocele, were observed after oral administration of halobetasol propionate during the period of organogenesis to pregnant rats and rabbits (see Data) . The available data do not support relevant comparisons of systemic halobetasol propionate exposures achieved in the animal studies to exposures observed in humans after topical use of DUOBRII Lotion. The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies. Data Human Data Available observational studies in pregnant women did not identify a drug-associated risk of major birth defects, preterm delivery, or fetal mortality with the use of topical corticosteroids of any potency. However, when the dispensed amount of potent or very potent topical corticosteroids exceeded 300 g during the entire pregnancy, maternal use was associated with an increased risk of low birth weight in infants. Animal Data Halobetasol propionate has been shown to cause malformations in rats and rabbits when given orally during organogenesis at doses of 0.04 to 0.1 mg/kg/day in rats and 0.01 mg/kg/day in rabbits. Halobetasol propionate was embryotoxic in rabbits but not in rats. Cleft palate was observed in both rats and rabbits. Omphalocele was seen in rats but not in rabbits. In an embryofetal development study in rats, a tazarotene gel formulation, 0.5% (0.25 mg/kg/day tazarotene) was topically administered to pregnant rats during gestation days 6 through 17. Reduced fetal body weights and reduced skeletal ossification occurred at this dose (11 times the MRHD based on AUC comparison). In an embryofetal development study in rabbits, a tazarotene gel formulation (0.5%, 0.25 mg/kg/day tazarotene) was topically administered to pregnant rabbits during gestation days 6 through 18. Single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies were noted at this dose (116 times the MRHD based on AUC comparison). When tazarotene was given orally to animals, developmental delays were seen in rats; malformations and post-implantation loss were observed in rats and rabbits at doses producing 9 and 228 times, respectively, the MRHD (based on AUC comparisons). In female rats orally administered 2 mg/kg/day of tazarotene from 15 days before mating through gestation day 7, classic developmental effects of retinoids including decreased number of implantation sites, decreased litter size, decreased numbers of live fetuses, and decreased fetal body weights were observed at this dose (16 times the MRHD based on AUC comparison). A low incidence of retinoid-related malformations was observed at that dose. In a pre- and postnatal development toxicity study, topical administration of a tazarotene gel formulation (0.125 mg/kg/day) to pregnant female rats from gestation day 16 through lactation day 20 reduced pup survival but did not affect the reproductive capacity of the offspring. Based on data from another study, the systemic drug exposure in the rat at this dose would be equivalent to 5 times the MRHD (based on AUC comparison). 8.2 Lactation Risk Summary There are no data on the presence of tazarotene, halobetasol propionate or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production after treatment with DUOBRII Lotion. After single topical doses of a 14 C-tazarotene gel formulation to the skin of lactating rats, radioactivity was detected in rat milk. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for DUOBRII Lotion and any potential adverse effects on the breastfed child from DUOBRII Lotion. Clinical Considerations Advise breastfeeding women not to apply DUOBRII Lotion directly to the nipple and areola to avoid direct infant exposure. 8.3 Females and Males of Reproductive Potential Pregnancy Testing DUOBRII Lotion is contraindicated in women who are pregnant. Females of reproductive potential should be warned of the potential risk and use adequate birth control measures during treatment with DUOBRII Lotion. The possibility that a female of reproductive potential is pregnant at the time of institution of therapy should be considered. A negative result for pregnancy should be obtained within 2 weeks prior to DUOBRII Lotion therapy, which should begin during menstruation. Contraception Based on animal studies, DUOBRII Lotion may cause fetal harm when administered to a pregnant female [see Use in Specific Populations (8.1) ] . Advise females of reproductive potential to use effective contraception during treatment with DUOBRII Lotion. 8.4 Pediatric Use Safety and effectiveness of DUOBRII Lotion in pediatric patients under the age of 18 years have not been evaluated. Because of higher skin-surface-area-to-body-mass ratios, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during or after withdrawal of treatment. Adverse reactions including striae have been reported with use of topical corticosteroids in infants and children [see Warnings and Precautions (5.2) ] . HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema [see Warnings and Precautions (5.2) ] . 8.5 Geriatric Use Of the 270 subjects exposed to DUOBRII Lotion in clinical trials, 39 subjects were 65 years or older. Clinical trials of DUOBRII Lotion did not include sufficient numbers of subjects age 65 years and older to determine whether they respond differently from younger subjects.
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING DUOBRII (halobetasol propionate and tazarotene) Lotion, 0.01%/0.045% is a white to off-white lotion supplied in a white aluminum tube as follows: • 100 g (NDC 0187-0653-01) Storage and Handling Conditions Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature] . Protect from freezing.
Learning Zones
The Learning Zones are an educational resource for healthcare professionals that provide medical information on the epidemiology, pathophysiology and burden of disease, as well as diagnostic techniques and treatment regimens.
Disclaimer
The drug Prescribing Information (PI), including indications, contra-indications, interactions, etc, has been developed using the U.S. Food & Drug Administration (FDA) as a source (www.fda.gov).
Medthority offers the whole library of PI documents from the FDA. Medthority will not be held liable for explicit or implicit errors, or missing data.
Drugs appearing in this section are approved by the FDA. For regions outside of the United States, this content is for informational purposes only and may not be aligned with local regulatory approvals or guidance.