News
NICE (UK) recommends avalglucosidase alfa to treat Pompe disease
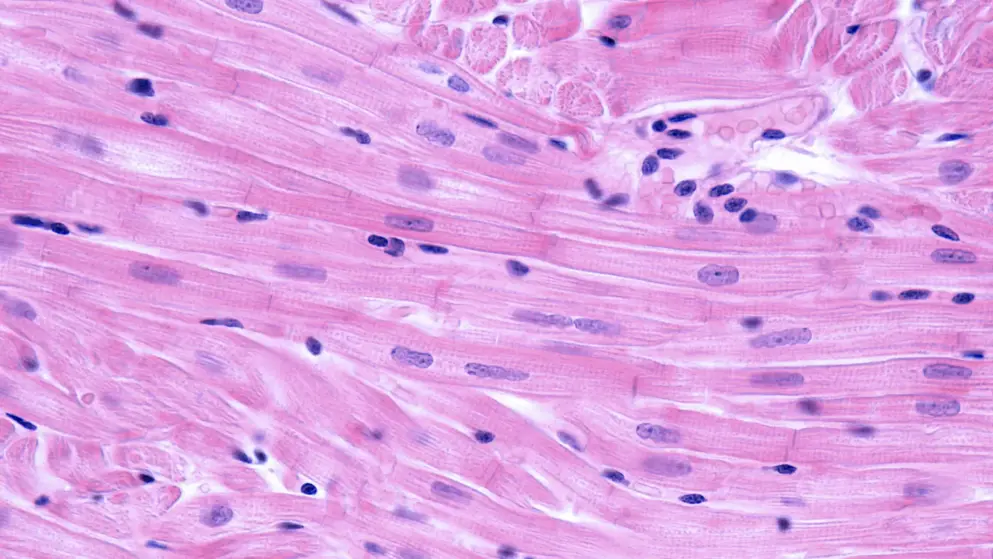
Avalglucosidase alfa from Sanofi has been recommended as an option for treating Pompe disease in babies, children, young people and adults
Pompe disease is caused by an enzyme deficiency that leads to a build-up of glyocogen in skeletal and heart muscles which causes muscle weakness. The treatment has been recommended as a long-term enzyme replacement therapy that aims to help reduce this glycogen build-up.
NICE and commissioning groups have agreed to provide funding to implement this recommendation 30 days after publication on 21 July (or when the product becomes commercially available). This is a shorter timescale than usual because it has been available already through the Early Access to Medicines Scheme (EAMS).
Condition: Pompe Disease
Type: drug