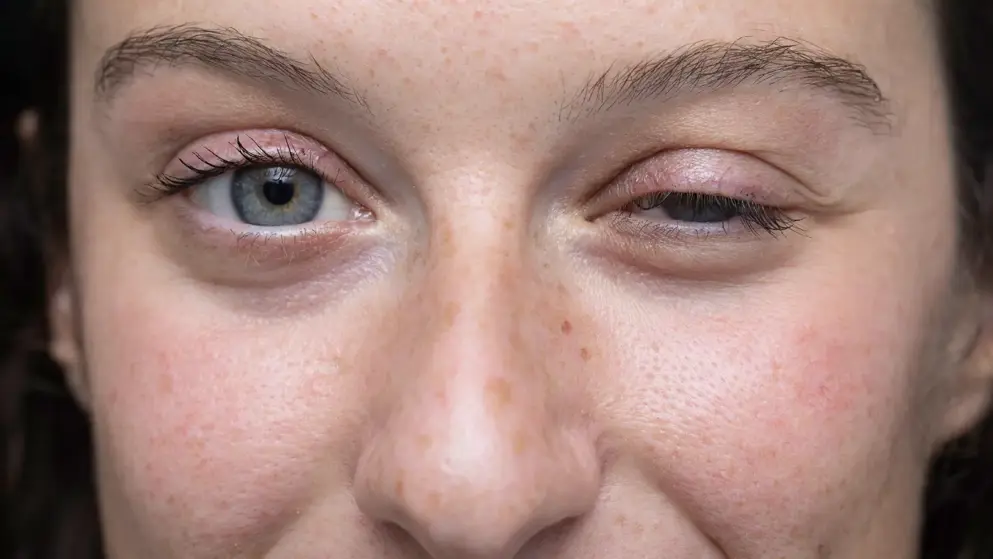
MHRA (UK) grants Early Access to Medicines Scheme availability for efgartigimod to treat generalised myasthenia gravis.- Argenx
MHRA (UK) grants Early Access to Medicines Scheme (EAMS) status to efgartigimod (Vyvgart) from Argenx, for the treatment of adult patients with AChR-antibody seropositive generalised myasthenia gravis (gMG), including patients with refractory gMG who have failed, not tolerated or are ineligible for licensed treatment.
Myasthenia gravis is a progressive, chronic neuromuscular disease that commonly strikes people between the ages of 40 and 70 and afflicts between 50,00 and 85,000 people in the United States. Approximately 13,600 new cases of myasthenia gravis are diagnosed each year.
The aim of the Early Access to Medicines Scheme (EAMS) is to provide earlier availability of promising new unlicensed medicines (medicines that do not have a marketing authorisation or are used outside their licence) to UK patients that have a high unmet clinical need. The medicines included in the scheme after they have received a positive scientific opinion are those that are intended to treat, diagnose or prevent seriously debilitating or life-threatening conditions where there are no adequate treatment options.