News
FDA extends review of avalglucosidase alfa, next-generation enzyme replacement therapy, for Pompe disease to 18 August 2021
The FDA has extended by three months its review of Sanofi Genzyme‘s application seeking approval of avalglucosidase alfa, its next-generation enzyme replacement therapy (ERT) for Pompe disease.
The FDA has extended by three months its review of Sanofi Genzyme‘s application seeking approval of avalglucosidase alfa, its next-generation enzyme replacement therapy (ERT) for Pompe disease. The date for an agency decision regarding approval, previously set for May 18, is now 18 August 2021.
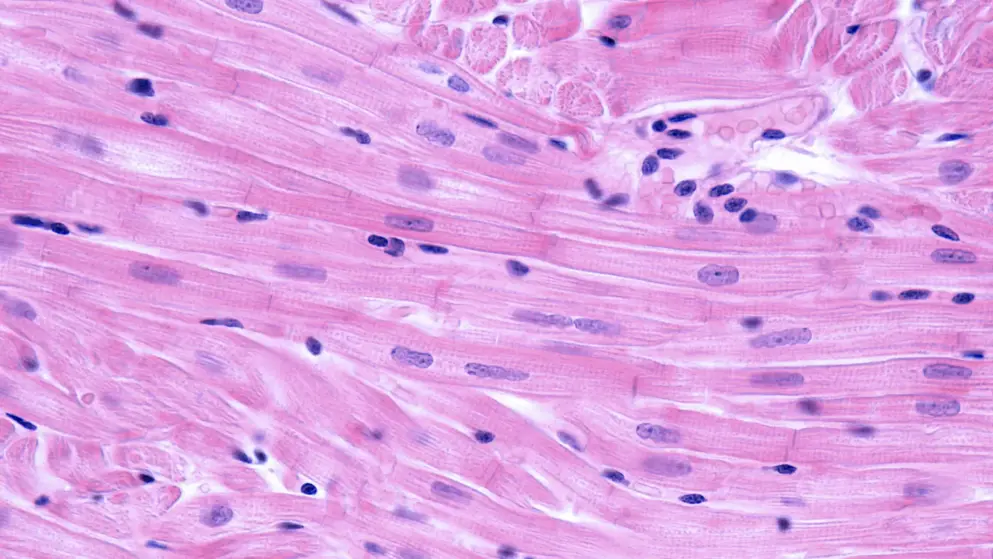
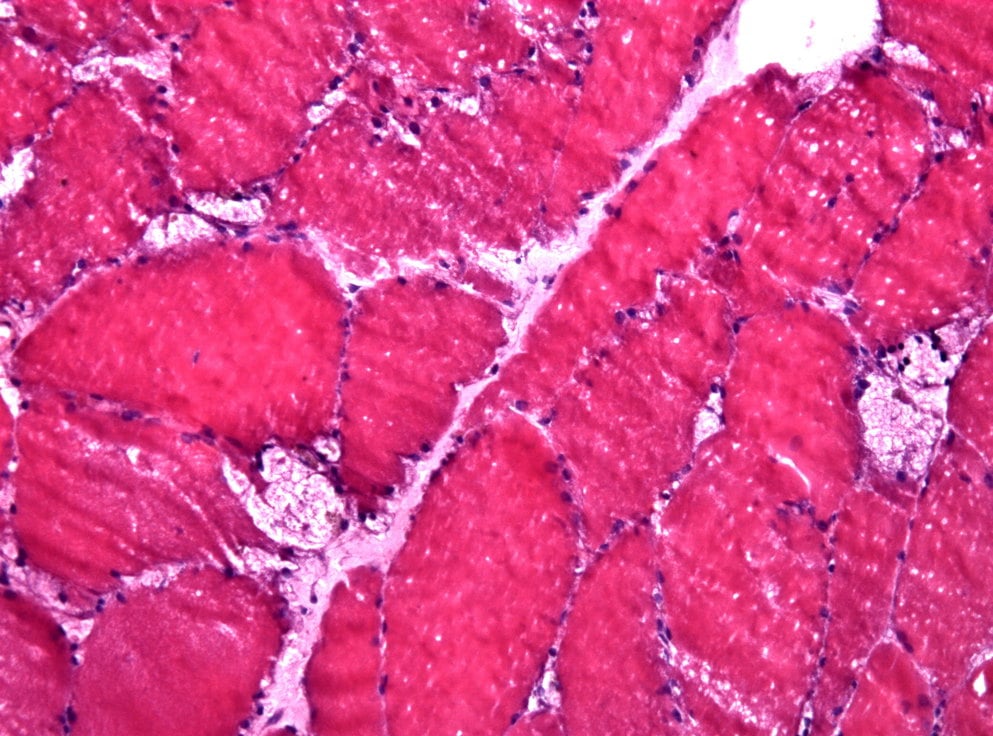
Avalglucosidase alfa is an investigational ERT intended to effectively clear glycogen — a sugar molecule whose abnormal buildup is a hallmark of Pompe disease — from target tissues. The potential therapy is also being reviewed by the European Medicines Agency, where a decision is expected in the second half of 2021..
Condition: Pompe Disease
Type: drug