News
Pfizer announces extension of review of NDA for abrocitinib for treatment of atopic dermatitis and for Xeljanz for ankylosing spondylitis.
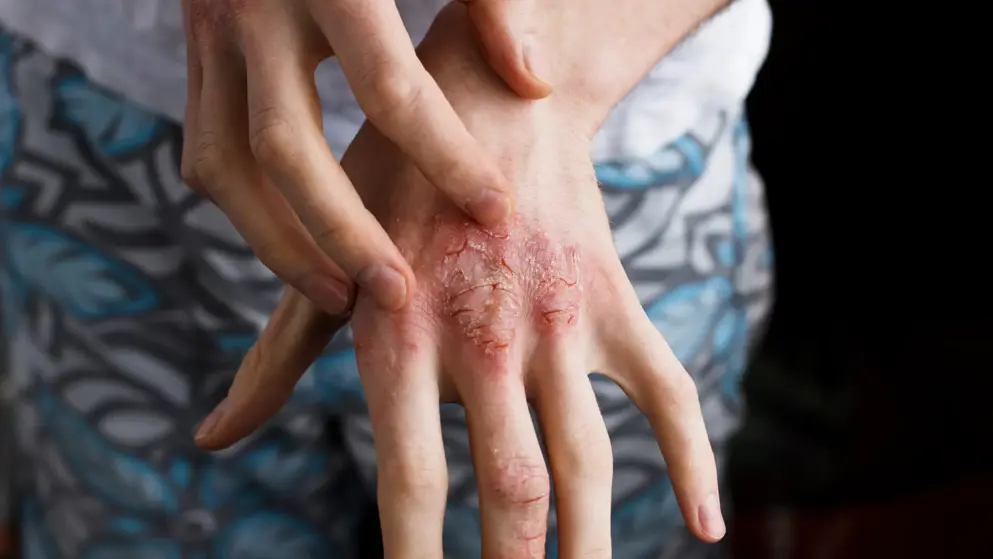
Pfizer Inc.has announced that the FDA has extended the priority review period for the New Drug Application (NDA) for abrocitinib for the treatment of adults and adolescents with moderate to severe atopic dermatitis. The Prescription Drug User Fee Act (PDUFA) goal date has been extended three months to early Q3 2021.
The FDA has also extended the review period for the Supplemental New Drug Applications (sNDAs) for Xeljanz / Xeljanz XR (tofacitinib) for the treatment of adults with active ankylosing spondylitis (AS) by three months, with a goal date in early Q3 2021.
Condition: Atopic Dermatitis (Eczema)
Type: drug