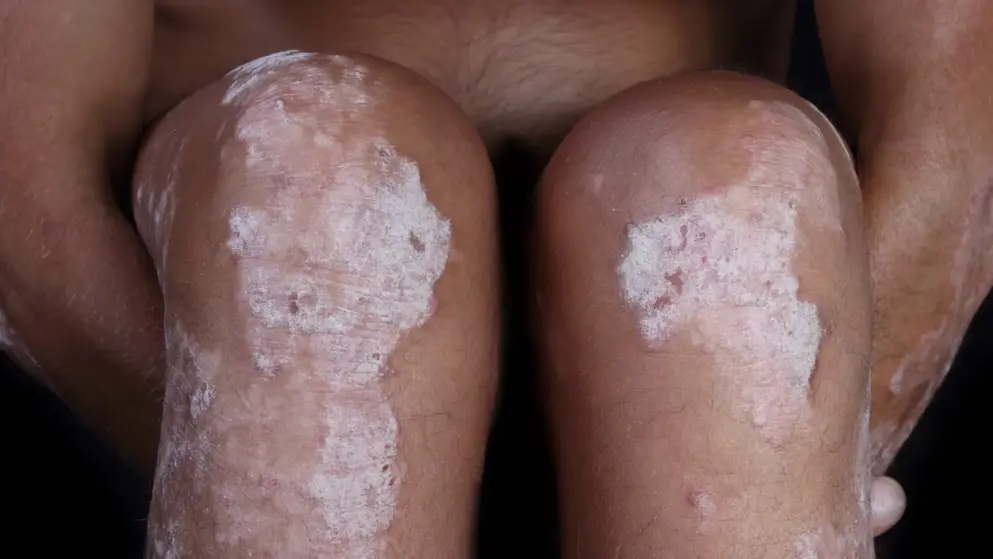
Arcutis submits topical roflumilast cream NDA to the FDA for the treatment of adults and adolescents with plaque psoriasis.
Roflumilast cream (ARQ-151) is a once-daily topical formulation of roflumilast, a highly potent and selective inhibitor of phosphodiesterase type 4 (PDE4), an enzyme that drives overactive immune responses. In clinical trials, roflumilast cream demonstrated robust efficacy coupled with favorable safety and tolerability that, if approved, would enable chronic use across the body, without many of the local tolerability issues associated with alternative treatments.
Topical treatments are the mainstay of therapy for the vast majority of psoriasis patients, particularly those with mild-to-moderate disease, as well as many moderate-to-severe patients who use topicals in combination with other treatments. However, existing topical treatments often force physicians and patients to make difficult trade-offs between tolerability and long-term use, requiring the use of multiple products or complicated treatment schedules. Roflumilast cream has been designed to address the challenges posed to dermatologists and patients by existing topical therapies and aims to simplify the overall management of plaque psoriasis...