News
TransCon hGH filed with EMA for pediatric growth hormone deficiency.- Ascendis Pharma
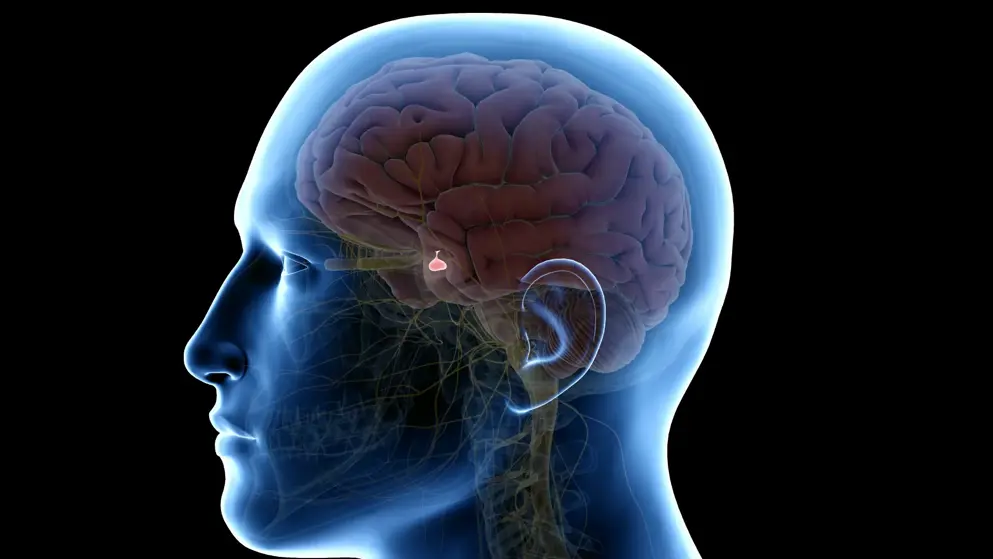
Ascendis Pharma announced the submission of a MAA to the EMA seeking approval for TransCon hGH (lonapegsomatropin), an investigational long-acting once-weekly prodrug of somatropin (human growth hormone or hGH), previously known as ACP 001, for the treatment of pediatric patients who are diagnosed with growth hormone deficiency (GHD). The MAA submission includes data from the clinical development program for TransCon hGH that included eight clinical trials evaluating safety and efficacy in more than 400 subjects with GHD.
Condition: Growth Hormone Deficiency
Type: drug