News
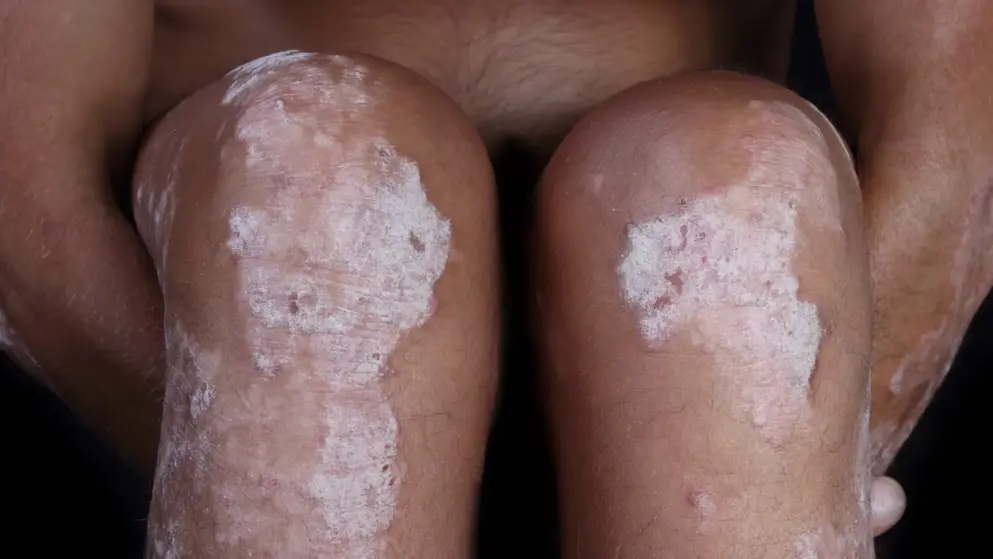
Mirikizumab superior to Cosentyx in a Phase III study for patients with moderate to severe plaque psoriasis.- Eli Lilly
Eli Lilly and Company announced that mirikizumab, an investigational monoclonal antibody that binds to the p19-subunit of IL23, met the primary and all key secondary endpoints versus placebo at Week 16 (superiority) and all key secondary endpoints versus Cosentyx (secukinumab) at Week 16 (non-inferiority) and Week 52 (superiority) in the OASIS-2 study.
OASIS-2 is a multicenter randomized, double-blind, placebo-controlled study comparing the efficacy and safety of mirikizumab to placebo and Cosentyx in patients with moderate to severe plaque psoriasis.
The safety profile was consistent with previously disclosed results for mirikizumab and known safety findings of other drugs in the IL23p19 class.
The full OASIS-2 study results will be disclosed at future congresses..
Condition: Psoriasis
Type: drug