Phase III trial evaluating Dupixent in atopic dermatitis meets primary endpoint.- Regeneron.
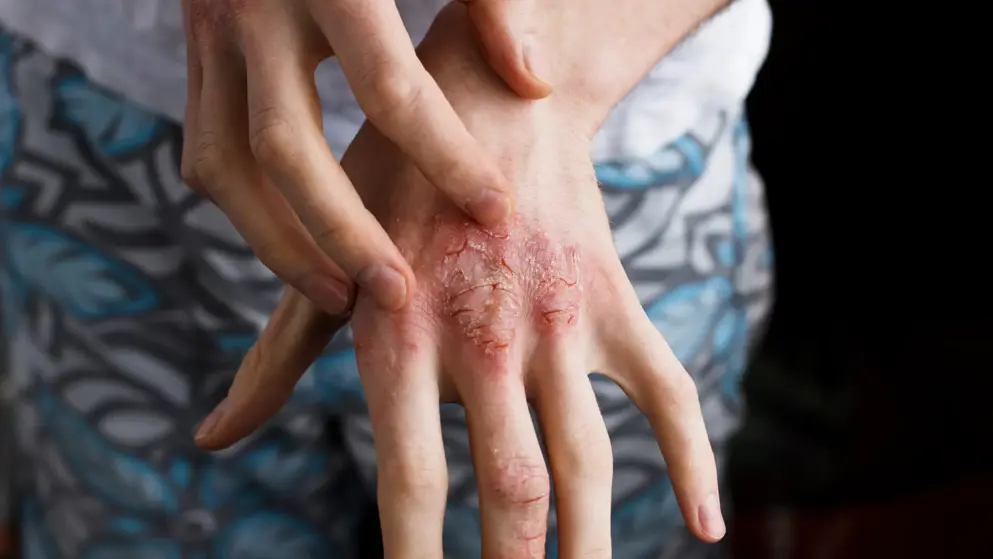
A pivotal Phase III trial evaluating Dupixent (dupilumab), from Regeneron, to treat moderate-to-severe atopic dermatitis in adolescents (ages 12-17) met its primary and key secondary endpoints. In the trial, treatment with Dupixent as monotherapy significantly improved measures of overall disease severity, skin clearing, itching, and certain health-related quality of life measures. Dupixent is the first and only biologic to show positive results in this patient population. The primary endpoints were the proportion of patients achieving Investigator's Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) and 75% improvement in Eczema Area and Severity Index (EASI-75, co-primary endpoint outside of the U.S.) at 16 weeks.
Results showed that 24% of patients who received weight-based dosing of Dupixent every two weeks (200 mg or 300 mg) and 18% of patients who received a fixed dose of Dupixent every four weeks (300 mg) achieved the primary endpoint - clear or almost-clear skin (IGA; score of 0 or 1) - compared with 2% with placebo. In addition, 41.5% of patients who received Dupixent every two weeks and 38% of patients who received Dupixent every four weeks achieved 75% or greater skin improvement (EASI-75) compared to 8% with placebo. There was also a 66% improvement in the Dupixent every two weeks group and, 65% improvement in the Dupixent every four weeks group in average percent change from baseline in EASI score compared with a 24% improvement in the placebo group. Finally, there was a 48% improvement in the Dupixent every two weeks group and 45.5% improvement in the Dupixent every four weeks group in average percent change from baseline in the pruritus numerical rating scale (NRS) compared with a 19% improvement in the placebo group.
For the 16-week treatment period, the overall rate of adverse events was comparable between the Dupixent groups and placebo (72% for Dupixent every two weeks, 64% for Dupixent every four weeks and 69% for placebo). There were no serious adverse events or events leading to treatment discontinuation in either Dupixent treatment group. Detailed results from this trial will be presented at a future medical meeting. These data will be submitted to regulatory authorities later this year.
Comment: Dupixent is approved in the U.S. for the treatment of adults with moderate-to-severe atopic dermatitis (eczema) that is not well controlled with prescription therapies used on the skin (topical), or who cannot use topical therapies. Dupixent can be used with or without topical corticosteroids. It is not known if Dupixent is safe and effective in children.