EU Commission approves Dupixent (dupilumab) to treat in adults with moderate-to-severe atopic dermatitis .- Sanofi + Regeneron.
Sanofi and Regeneron Pharmaceuticals, Inc. announced that the European Commission has granted marketing authorization for Dupixent (dupilumab), for use in adults with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy.
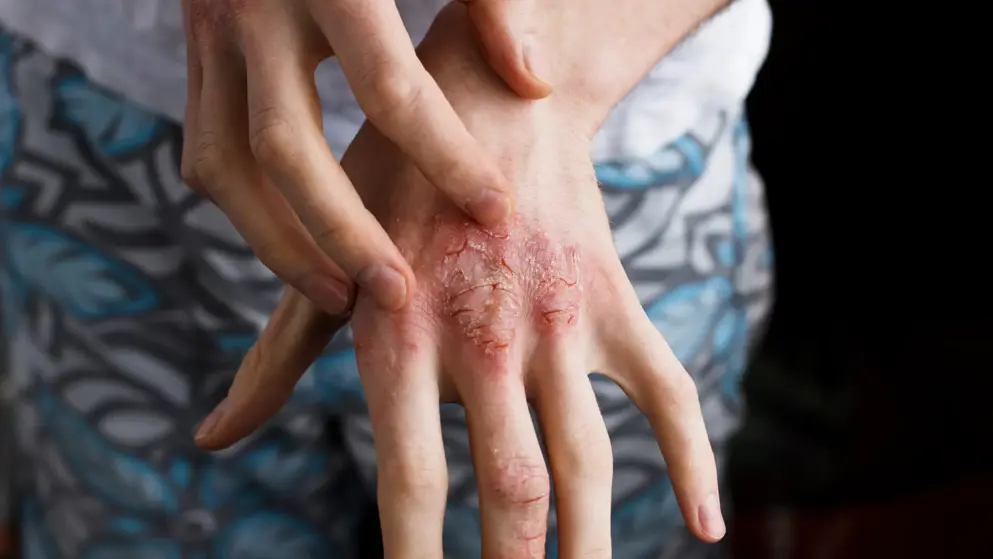
Atopic dermatitis, a form of eczema, is a chronic inflammatory disease with symptoms often appearing as a rash on the skin. Moderate-to-severe atopic dermatitis is characterized by rashes often covering much of the body, and can include intense, persistent itching and skin dryness, cracking, redness, crusting, and oozing. Anchor Itch is one of the most burdensome symptoms for patients and can be debilitating. In addition, people with moderate-to-severe atopic dermatitis experience impaired quality of life, including disrupted sleep, and increased anxiety and depression symptoms along with their disease.
Comment: Dupixent is approved in the U.S. for the treatment of adults with moderate-to-severe atopic dermatitis (eczema) that is not well controlled with prescription therapies used on the skin (topical), or who cannot use topical therapies. Dupixent can be used with or without topical corticosteroids. It is not known if Dupixent is safe and effective in children.