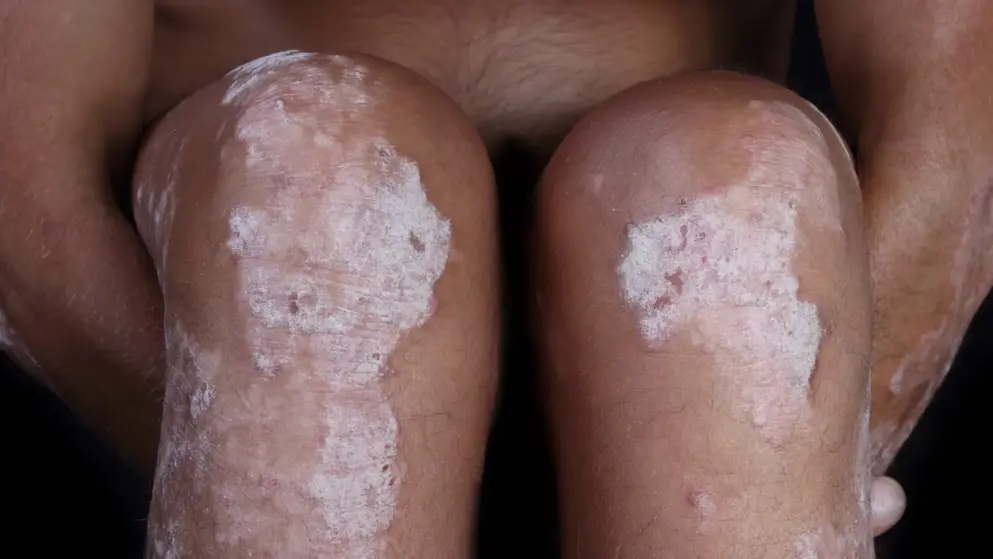
CHMP recommends approval of Tremfya (guselkumab) in the treatment of adults with moderate to severe plaque psoriasis.- Janssen-Cilag.
Janssen-Cilag International NV announced that the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending marketing authorisation in the European Union for the use of Tremfya (guselkumab) in the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy.
The EU filing is based primarily on data from three Phase III clinical studies. The VOYAGE 1 and VOYAGE 2 trials demonstrated the superior efficacy of guselkumab across all primary and major secondary endpoints compared with placebo and HUMIRA (adalimumab). Results showed high levels of skin clearance after just 16 weeks, with a 90% reduction in Psoriasis Area and Severity Index (PASI 90) score in 73.3% and 70.0% of patients receiving guselkumab, compared with 49.7% and 46.8% of patients receiving adalimumab in the VOYAGE 1 and 2 trials, respectively. In the VOYAGE 1 and 2 trials, 27.0% and 27.3% of patients receiving guselkumab, respectively, achieved a symptom score of zero (symptom-free) at week 16 compared with less than 1% of patients in the placebo groups and 16.5% and 15.0% in the adalimumab groups. In addition, the NAVIGATE trial demonstrated that patients who did not achieve a response of cleared or minimal disease (Investigator's Global Assessment score of 0 or 1) by week 16 when treated with STELARA (ustekinumab), significantly benefited when switched to guselkumab.
Comment: Janssen received US FDA approval of guselkumab for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy in July 2017.