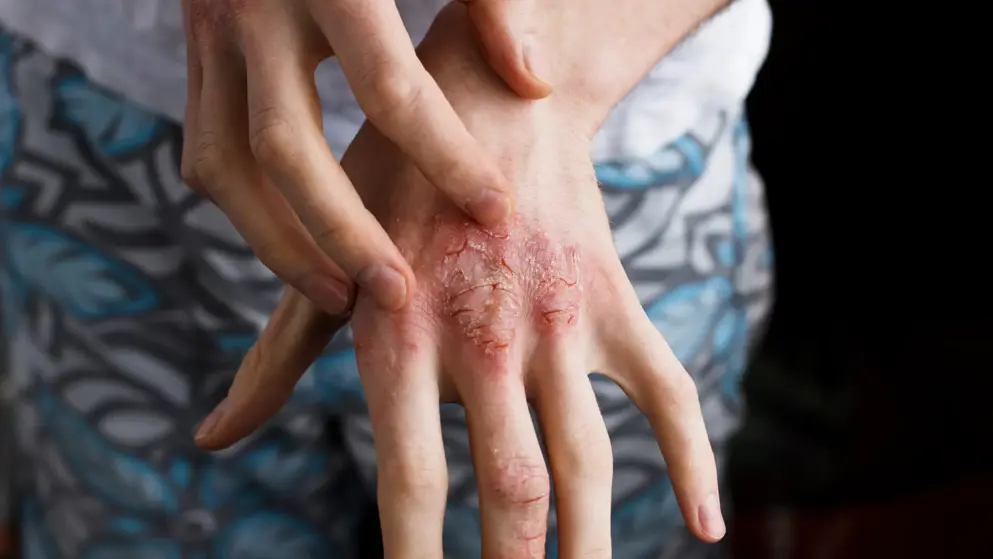
Roche licenses lebrikizumab to Demira for atopic dermatitis and all other indications - except for interstitial lung diseases.
Dermira will obtain exclusive, worldwide rights to develop and commercialize lebrikizumab, a monoclonal antibody targeting interleukin 13 (IL-13), for atopic dermatitis and all other indications - except for interstitial lung diseases, such as idiopathic pulmonary fibrosis, the rights for which will remain with Roche.
Dermira will make an initial payment of $80 million to Roche and payments totaling $55 million in 2018. Dermira will also be obligated to make additional payments upon the achievement of certain milestones: $40 million on the initiation of Dermira's first Phase III clinical study, up to $210 million for regulatory and first commercial sale milestones in certain territories and up to $1.025 billion based on the achievement of certain thresholds for net sales of lebrikizumab for indications other than interstitial lung disease.