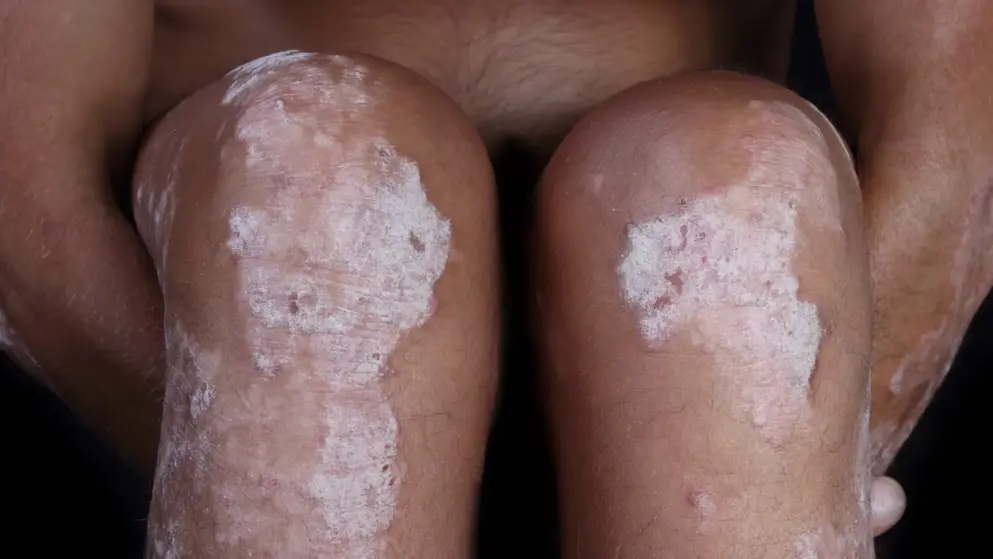
Stelara (ustekinumab) filed with the FDA for the treatment of adolescents (12 to 17 years of age) with moderate to severe plaque psoriasis- Janssen Biotech
Janssen Biotech announced the submission of a Supplemental Biologics License Application (sBLA) to the FDA seeking approval of Stelara (ustekinumab) for the treatment of adolescents (12 to 17 years of age) with moderate to severe plaque psoriasis. Stelara a human monoclonal antibody that targets interleukin (IL)-12 and IL-23 cytokines, has been approved in the United States for the treatment of adults with moderate to severe plaque psoriasis since September 2009.
The application is supported by data from the Phase III CADMUS registration study, which evaluated the efficacy and safety of Stelara in the treatment of adolescents (12 to 17 years of age) with moderate to severe plaque psoriasis. Results from the CADMUS study have been previously published in the Journal of the American Academy of Dermatology in May 2015. The efficacy and safety profile of Stelara in the CADMUS trial was consistent with the profile of this anti-IL-12/23 monoclonal antibody as previously observed in adult patients receiving Stelara.