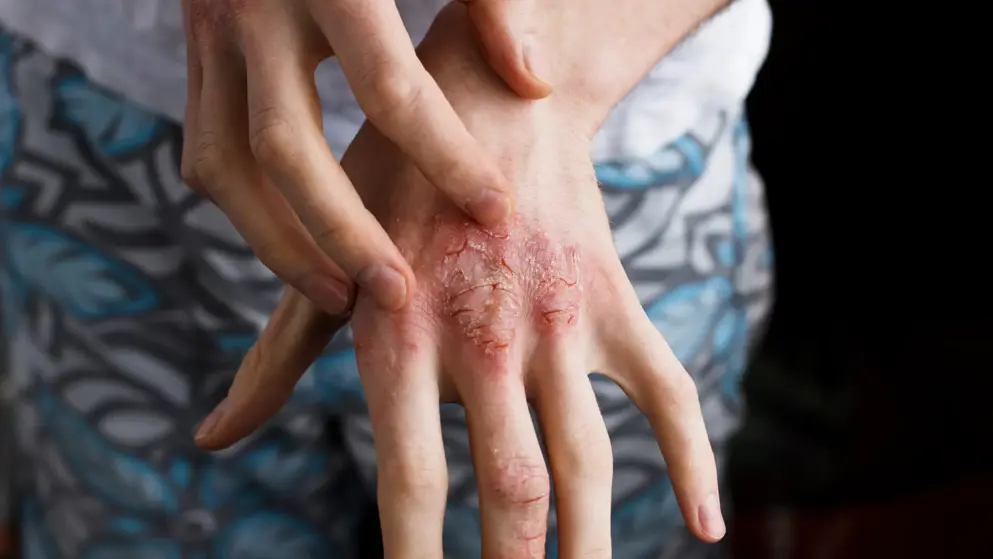
Dupilumab success in LIBERTY AD CHRONOS trial for treatment of atopic dermatitis.- Sanofi + Regeneron
Sanofi and Regeneron Pharmaceuticals, Inc. announced that a one-year Phase III study, known as LIBERTY AD CHRONOS, evaluating investigational dupilumab met its primary and key secondary endpoints. In the study, dupilumab with topical corticosteroids (TCS) was compared to TCS alone in moderate-to-severe atopic dermatitis (AD) adult patients. Patients enrolled in the study were inadequately controlled by topical corticosteroids (TCS) with or without topical calcineurin inhibitor (TCI). Dupilumab with TCS significantly improved measures of overall disease severity at 16 and 52 weeks, when compared to placebo with TCS.
The primary endpoint results at week 16 were the following: 39 percent of patients who received either dupilumab 300 mg weekly or dupilumab 300 mg every two weeks with TCS achieved clearing or near-clearing of skin lesions (IGA 0 or 1), compared to 12 percent of patients receiving placebo with TCS (p less than 0.0001).64 percent of patients who received dupilumab 300 mg weekly with TCS, and 69 percent of patients who received dupilumab 300 mg every two weeks with TCS achieved EASI-75, compared to 23 percent of patients receiving placebo with TCS (p less than 0.0001).
The secondary endpoint 52-week results were the following: 40 percent of patients who received dupilumab 300 mg weekly with TCS, and 36 percent of patients who received dupilumab 300 mg every two weeks with TCS achieved clearing or near-clearing of skin lesions (IGA 0 or 1), compared to 12.5 percent of patients receiving placebo with TCS (p less than 0.0001). 64 percent of patients who received 300 mg weekly with TCS, and 65 percent of patients who received 300 mg every two weeks with TCS achieved EASI-75, compared to 22 percent with placebo with TCS (p less than 0.0001). Patients were less likely to discontinue therapy in the dupilumab with TCS groups compared to placebo with TCS group (15 percent in both dupilumab groups; 33 percent placebo).
The overall rate of adverse events was comparable between the dupilumab with TCS groups (83 percent for the weekly dose and 88 percent for the every two weeks dose) and the placebo with TCS group (84 percent). The rate of serious adverse events was comparable between the dupilumab with TCS groups (3 and 4 percent) and placebo with TCS group (5 percent). Serious and/or severe infections were numerically higher in the placebo with TCS group (1 percent in both dupilumab groups and 2 percent placebo).