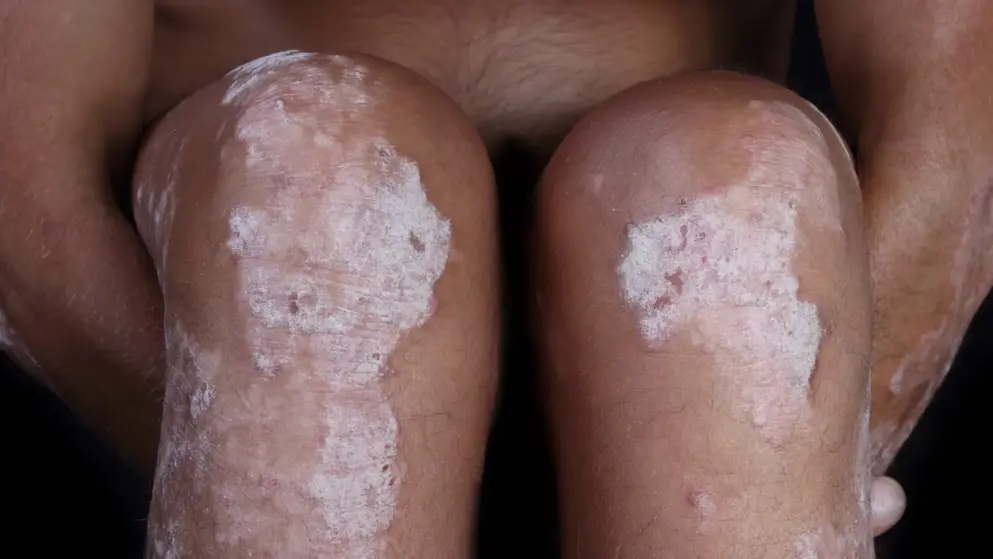
Ixekizumab superior to etanercept and placebo in UNCOVER-2 and UNCOVER-3 Phase III trials for psoriasis- Eli Lilly
Eli Lilly and Company announced that treatment for moderate-to-severe plaque psoriasis with ixekizumab resulted in clinically meaningful improvements as early as one week, compared to patients treated with etanercept or placebo. Detailed results of this combined analysis of UNCOVER-2 and UNCOVER-3 were presented during the American Academy of Dermatology (AAD) Annual Meeting taking place March 4-8 in Washington, D.C.
UNCOVER-2 and UNCOVER-3 are double-blind, multicenter, Phase III studies evaluating more than 2,500 patients with moderate-to-severe plaque psoriasis across 19 countries. In these comparator studies, patients were assigned to receive either placebo, etanercept (50 mg twice a week) or ixekizumab (80 mg every two or four weeks) for 12 weeks, following a 160-mg starting dose. This combined analysis evaluated the speed of onset of clinical improvement as measured by mean percentage improvement in Psoriasis Area Severity Index (PASI) score from baseline, as well as time to PASI 50 and PASI 75 among patients treated with ixekizumab, etanercept or placebo. PASI measures the extent and severity of psoriasis by assessing average redness, thickness and scaliness of skin lesions (each graded on a zero to four scale), weighted by the body surface area of involved skin.
Significant differences in mean percentage improvement of psoriasis plaques were observed among patients treated with ixekizumab compared to etanercept and placebo: At one week, the mean percentage improvement was 32.7 percent in the group randomized to receive ixekizumab every two weeks, 10.3 percent in etanercept and 5.31 percent in placebo (p < 0.001 for all comparisons). At two weeks, the mean percentage improvement was 53.6 percent in the group randomized to receive ixekizumab every two weeks, 23.3 percent in etanercept and 9.25 etanercept in placebo (p < 0.001 for all comparisons). Treatment with ixekizumab also resulted in clinically meaningful improvements (PASI 50) as early as one week, which were statistically significantly different compared with etanercept and placebo. At one week, PASI 50 was achieved by 22.8 percent of patients treated with ixekizumab every two weeks compared to 3.9 percent among those treated with etanercept and 1.4 percent in placebo (p < 0.001 for all comparisons). At two weeks, PASI 50 was achieved among 58.8 percent of patients treated with ixekizumab every two weeks compared to 14.6 percent of those treated with etanercept and 4.2 percent in placebo (p < 0.001 for all comparisons). Median time to PASI 75 was 30 days among patients treated with ixekizumab every two weeks and 85 days among those treated with etanercept. The majority of treatment-emergent adverse events were mild or moderate.
Comment: Ixekizumab (provisionally Taltz) was filed at the FDA in January 2016 and received CHMP recommendation in February 2016.