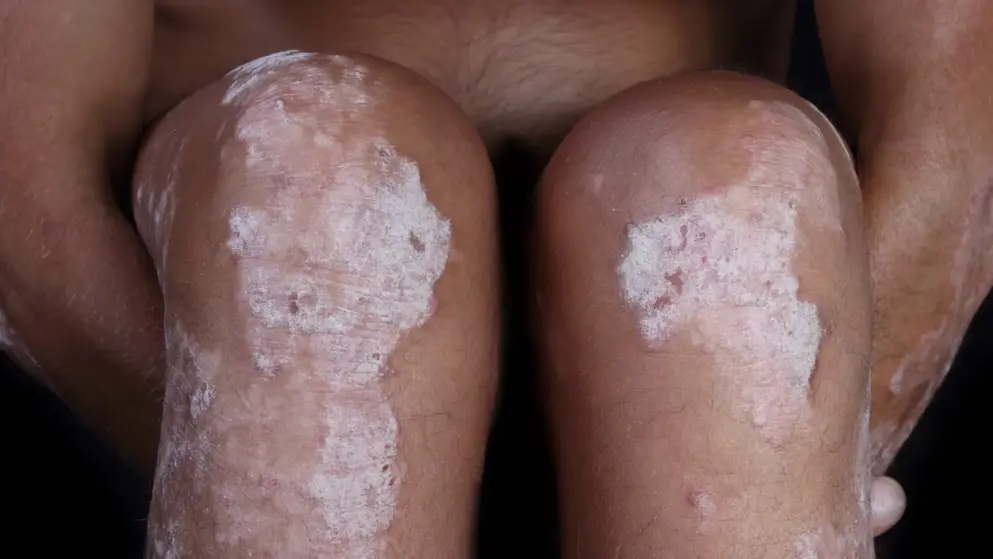
NICE draft guidance supports use of Cosentyx (secukinumab) in severe plaque psoriasis- Novartis
NICE (National Institute for Health and Care Excellence) in the UK, presented final draft guidance that Cosentyx (secukinumab) from Novartis can be used to treat adults with severe plaque psoriasis. Cosentyx is EU approved for first line treatment of the condition. Other treatments, such as TNF inhibitors and anti IL 12/23 Stelara, have EU approvals as second line treatment. NICE however will require patients to have tried other treatments e.g. ciclosporin, methotrexate and PUVA and ultra violet radiation before treatment with Cosentyx, if they are suitable.
Comment: Cosentyx has a different mechanism of action to current therapies- a fully human monoclonal antibody neutralizing interleukin-17A, a key pro-inflammatory cytokine- and has shown evidence of cases where patients experienced complete clearance of the condition. It has been shown superior to Enbrel and placebo in clinical trials and has less side effects than its competitors.