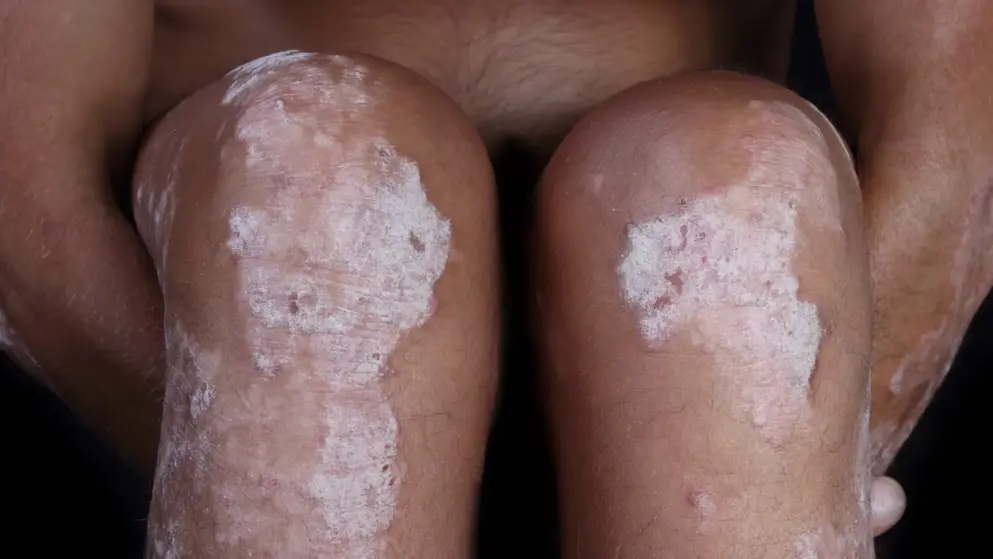
Leo Pharma files Enstilar (calcipotriol/betamethasone dipropionate), a cutaneous foam, in EU for treatment of Psoriasis
Leo Pharma A/S has announced it submitted a Marketing Authorisation Application to 30 European Health Authorities for Enstilar (calcipotriol/betamethasone dipropionate 50 micrograms/g / 0,5 mg/g) cutaneous foam for the treatment of psoriasis vulgaris, a chronic, inflammatory skin disease that can significantly impact quality of life for patients. Enstilar has the potential to be the first fixed combination cutaneous foam approved for the treatment of psoriasis vulgaris. The new cutaneous foam formulation is a fixed combination of calcipotriol and betamethasone (as dipropionate) developed with the aim of improving treatment for patients with psoriasis vulgaris – the most common form of psoriasis.
The European submission is based on the phase IIIa trial, the PSO-FAST trial, which evaluated the efficacy, safety, itch relief and improvement of itch-related sleep loss across a four week period with Enstilar as well as the phase 2 maximum use systemic exposure safety study of Enstilar.
> In December 2014, a New Drug Application (NDA) was submitted to the FDA for Enstilar.