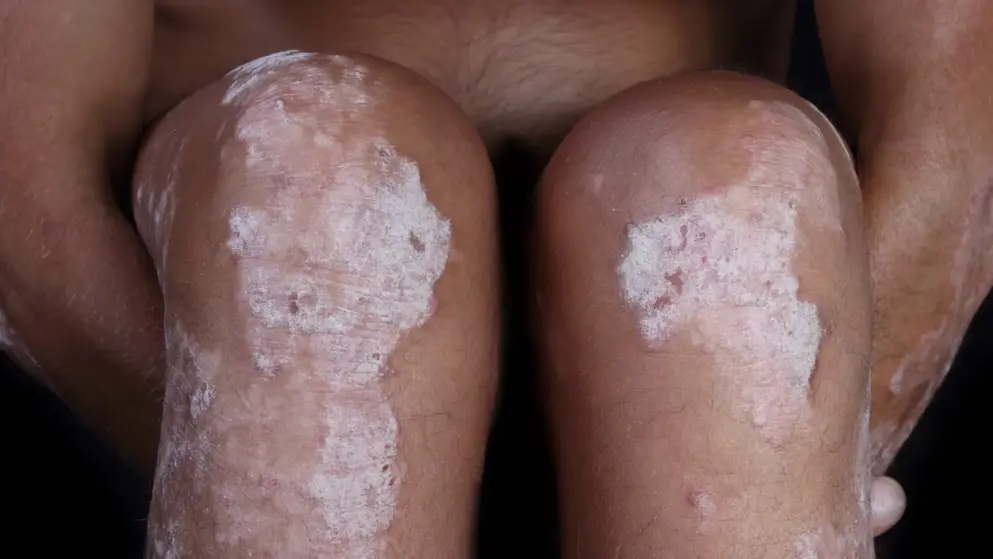
Apremilast (Celgene) success in Psoriasis
Celgene International S'rl, announced results of pre-specified sub-analyses from ESTEEM 1 on nail and scalp Psoriasis, as well as health-related quality-of-life outcomes, from the Company's first phase III study in Psoriasis, at the 22nd Congress of the European Academy of Dermatology and Venereology annual meeting in Istanbul, Turkey.
ESTEEM 1 is the largest of two registrational, randomized, placebo-controlled studies evaluating apremilast, an oral small-molecule specific inhibitor of phosphodiesterase 4 (PDE4), in more than 1,200 patients with moderate-to-severe plaque Psoriasis. Previously reported findings from ESTEEM 1 showed that apremilast significantly improved general signs and symptoms of Psoriasis across a wide-range of patient types.
After 16 weeks of treatment, patients in the apremilast 30 mg twice daily (BID) group had significantly greater improvements in the Nail Psoriasis Severity Index (NAPSI) scores than the patients treated with placebo, showing an improvement of 22.5% vs. a worsening of 6.5%, respectively; P<0.0001. improvements continued through 32 weeks of treatment for those patients on apremilast 30 mg bid (an improvement of 43.6%).></0.0001.>
Psoriasis of the scalp, another difficult-to-treat area, was also improved by treatment with apremilast 30 mg BID. After 16 weeks of therapy, significantly more patients treated with apremilast 30 mg achieved a ScPGA score of 0-1 (clear or almost clear) compared with those in the placebo group (46.5% vs. 17.5%, respectively; P<0.0001). this effect was generally maintained for those patients who remained on apremilast through week 32.></0.0001).>
As shown in a separate analysis (abstract #0237), the treatment of 844 patients in ESTEEM 1 with apremilast also significantly improved health-related quality-of-life, as assessed by a variety of standardized measurements, including the Dermatology Quality of Life Index (DLQI), the Patient Health Questionnaire (PHQ-8), the European Quality of Life 5 Dimensions Questionnaire (EQ-5D), and the 36-item Short-Form Health Survey (SF-36) mental component summary (MCS.)