Type 2 Inflammation
What is Type 2 Inflammation?
Type 2 inflammation is a common driver for underlying pathophysiology across multiple inflammatory diseases, including asthma and chronic rhinosinusitis with nasal polyps (CRSwNP)1
Involving both the adaptive and innate arms of the immune system, type 2 inflammation is characterized by key cytokines, particularly interleukin (IL)-4, IL-13, and IL-5 (FIGURE 1).1,2
FIGURE 1: Type 2 Inflammation Is Driven by Both the Adaptive and Innate Arms of the Immune System.1,3-8 *Downstream from Th2 cells, innate cells (such as mast cells and basophils) can also play a role in adaptive immunity given their activation by antigen-bound IgE. Crosslinking of IgE on these cells leads to release of several inflammatory mediators (including IL-4 and IL-13), thus amplifying the type 2 inflammatory response.1 IgE, immunoglobulin E; ILC2, type 2 innate lymphoid cell; Th2, T helper type 2.
Underlying type 2 inflammation plays a role across multiple diseases in a range of organ systems (FIGURE 2).1,9-18
FIGURE 2: Type 2 Inflammation Plays a Role Across Multiple Diseases.1,9-18 AERD, aspirin-exacerbated respiratory disease; COPD, chronic obstructive pulmonary disease; NSAID-ERD, non-steroidal anti-inflammatory drug-exacerbated respiratory disease.
Burden of Disease
Type 2 inflammatory diseases, including asthma and CRSwNP, are associated with substantial disease-specific signs, symptoms, and impaired quality of life (FIGURE 3).14,19-21
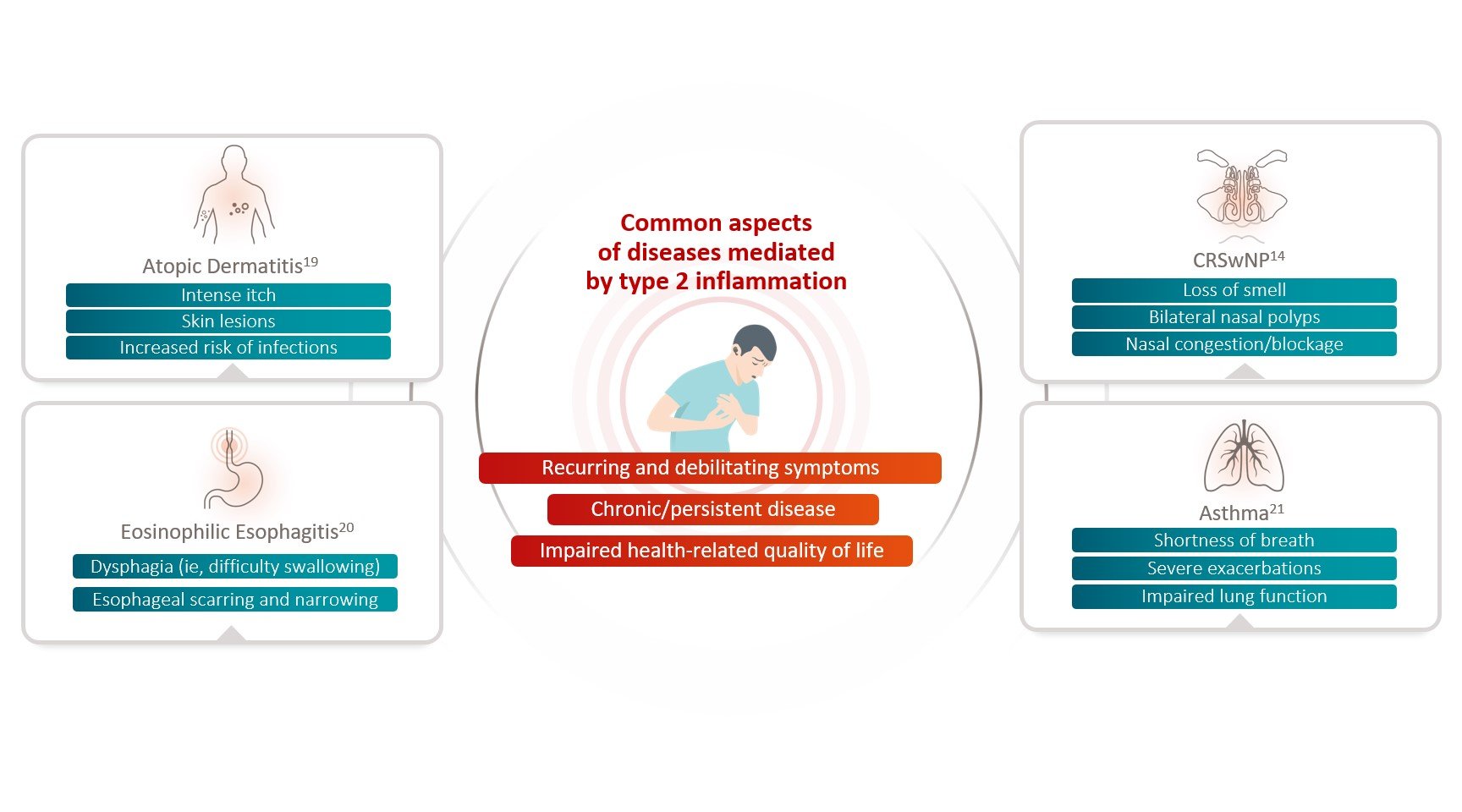
FIGURE 3: Common Aspects of Diseases Mediated by Type 2 Inflammation.14,19-21
Pathophysiology
Multiple type 2 inflammatory diseases such as asthma, CRSwNP, atopic dermatitis, and eosinophilic esophagitis have a common underlying pathophysiology.1
Type 2 Inflammation: Pathophysiologic Features That Underlie Multiple Inflammatory Diseases
Watch this short video to learn about the common pathophysiology underlying type 2 inflammatory diseases, including asthma and CRSwNP. Clinical manifestations from this shared pathophysiology and the additional burden from coexisting type 2 inflammatory diseases are also presented, supporting the key and central role of IL-4 and IL-13 in type 2 inflammation across multiple diseases.
Unifying the Heterogeneous Pathophysiology of Type 2 Inflammatory Diseases
Hear Prof. Oscar Palomares discuss the latest evidence in type 2 inflammation, unifying the heterogeneous pathophysiology of type 2 inflammatory diseases.
IL-4 and IL-13 are key drivers of systemic and local tissue inflammation and mediate different pathophysiologic consequences across type 2 inflammatory diseases (FIGURE 4).1,11,14,21-2
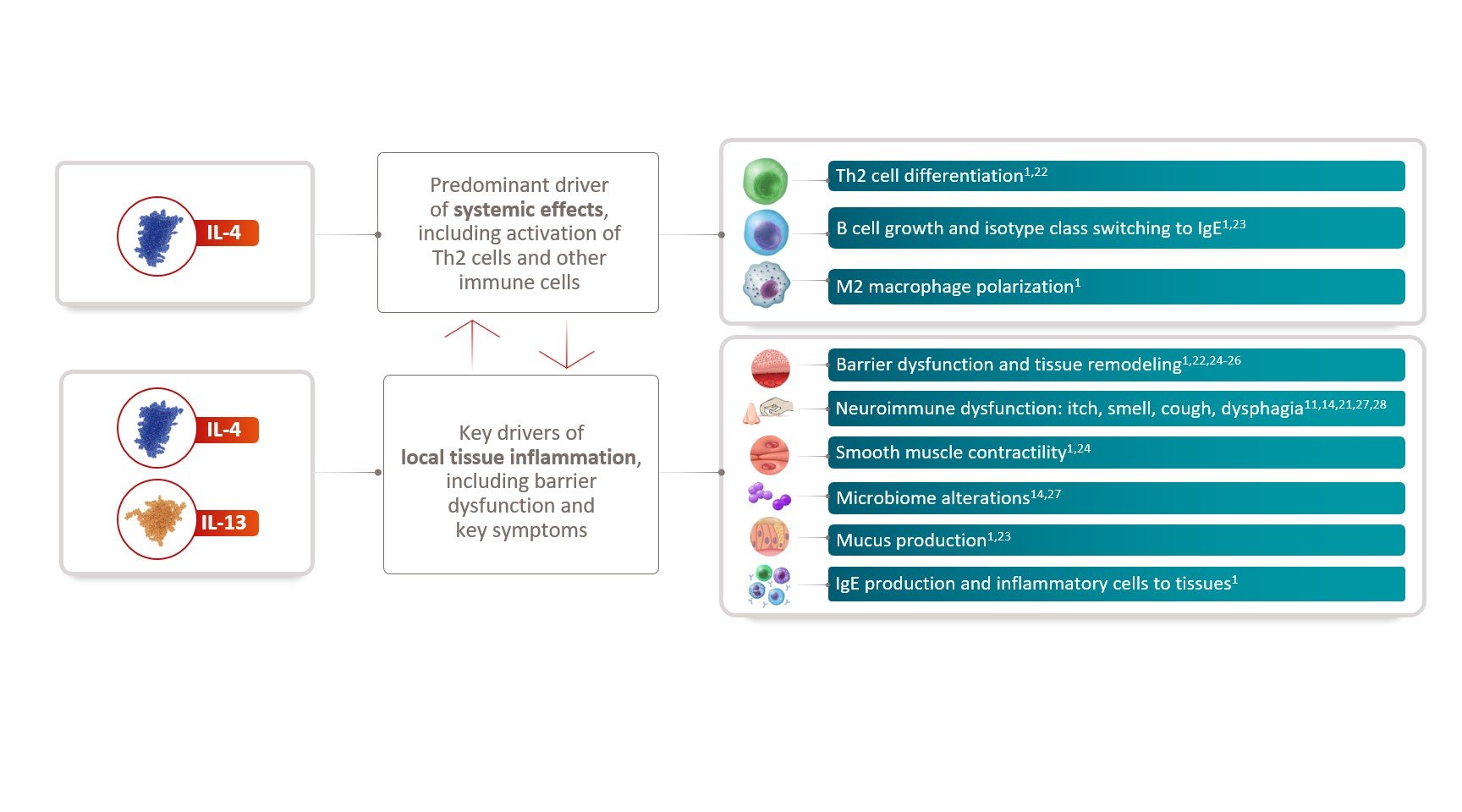
FIGURE 4: IL-4 and IL-13 Are Key Drivers of Systemic and Local Tissue Inflammation.1,11,14,21-28
Identifying Type 2 Inflammation
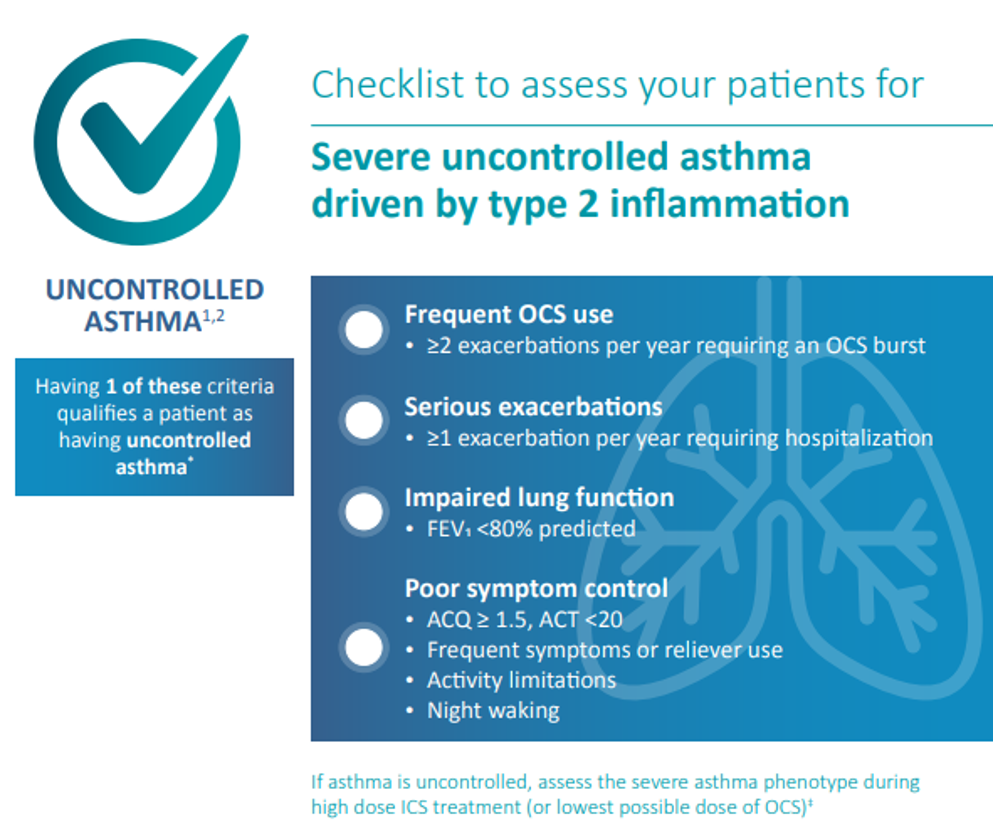
Recognizing features of type 2 inflammation plays an important role in identification and diagnosis of respiratory diseases such as asthma and CRSwNP, which are predominantly driven by type 2 inflammation.1,29,30
Type 2 inflammation is the most common endotype of inflammation in CRSwNP, driving disease pathology in up to 87% of patients (FIGURE 5).29,30
FIGURE 5: CRS With or Without Nasal Polyps Diagnosis Is Based on Symptoms and Objective Evidence of Disease.31 CRS, chronic rhinosinusitis; EPOS, European Position Paper on Rhinosinusitis; NP, nasal polyps.
Visit ADVENTprogram.com for additional resources on type 2 inflammatory diseases
You are leaving an independent site to a non-independent site.
References
- Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat RevDrug Discov.2016;15(1):35-50.
- Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. NEnglJ Med. 2017;377(10):965-976.
- Gandhi NA,PirozziG, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425-437.
- Ahern S, Cervin A. Inflammation and endotyping in chronic rhinosinusitis-A paradigm shift. Medicina (Kaunas).2019;55(4):95.
- Kim DW, Cho SH. Emerging endotypes of chronic rhinosinusitis and its application to precision medicine. Allergy Asthma Immunol Res.2017;9(4):299-306.
- Kariya S, Okano M, Nishizaki K. Biological basis and clinical implications of immunological molecules involved in eosinophilic inflammation in allergicrhinitis, chronic rhinosinusitis, and asthma. Advan Cell Molec Otolaryngol. 2015;3(1):26601.
- Mahdavinia M, Carter RG, Ocampo CJ, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014;133(6):1759-1763.
- Gibbs BF, Patsinakidis N, Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front Immunol. 2019;10:1383.
- Teraki Y, Hotta T, Shiohara T. Skin-homing interleukin-4 and -13-producing cells contribute to bullous pemphigoid: remission of disease is associatedwith increased frequency of interleukin-10-producing cells. J Invest Dermatol. 2001;117(5):1097-1102.
- Fukushi S, Yamasaki K, Aiba S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br J Dermatol. 2011;165(5):990-996.
- Davis BP, Rothenberg ME. Mechanisms of disease of eosinophilic esophagitis. Annu Rev Pathol. 2016;11:365-393.
- Johnston LK, Chien KB, Bryce PJ. The immunology of food allergy. J Immunol. 2014;192(6):2529-2534.
- Bachert C, Pawankar R, Zhang L, et al. ICON: chronic rhinosinusitis. World Allergy Organ J. 2014;7(1):25.
- Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331-357.
- Kowalski ML. Heterogeneity of NSAID-exacerbated respiratory disease: has the time come for subphenotyping?. Curr Opin Pulm Med. 2019;25(1):64-70.
- Garudadri S, Woodruff PG. Targeting chronic obstructive pulmonary disease phenotypes, endotypes, and biomarkers. Ann Am Thorac Soc. 2018;15(suppl 4):S234-S238.
- Rathore VP, Johnson B, Fink JN, Kelly KJ, Greenberger PA, Kurup VP. T cell proliferation and cytokine secretion to T cell epitopes of Asp f 2 in ABPA patients. Clin Immunol. 2001;100(2):228-235.
- Kurup VP, Seymour BW, Choi H, Coffman RL. Particulate Aspergillus fumigatus antigens elicit a TH2 response in BALB/c mice. J Allergy Clin Immunol. 1994;93(6):1013-1020.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109-1122.
- Hill DA, Spergel JM. The immunologic mechanisms of eosinophilic esophagitis. Curr Allergy Asthma Rep. 2016;16(2):9.
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Updated 2021. Accessed April 21, 2022.https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf
- Furue M. Regulation of skin barrier function via competition between AHR Axis versus IL-13/IL-4‒JAK‒STAT6/STAT3 axis: pathogenic and therapeutic implications in atopic dermatitis. J Clin Med. 2020;9(11):3741.
- Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57-65.
- O'Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology. 2018;154(2):333-345.
- Doucet C, Brouty-Boyé D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B. IL-4 and IL-13 specifically increase adhesion molecule andinflammatory cytokine expression in human lung fibroblast. Int Immunol. 1998;10(10):1421-1433.
- Doucet C, Brouty-Boyé D, Pottin-Clémenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma.J Clin Invest. 1998;101(10):2129-2139.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345-360.
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD.Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1.
- Stevens WW, Peters AJ, Tan BK, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. JAllergy Clin Immunol Pract. 2019;7(8):2812-2820.e3.
- Wang X, Zhang N, Bo M, et al.Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138(5):1344-1353.
- Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1-464.
Developed by EPG Health for Medthority in collaboration with Sanofi and Regeneron, with content provided by Sanofi and Regeneron.
ADVENT is a medical education non-promotional program for healthcare professionals organized by Sanofi and Regeneron. This website is intended only for duly authenticated healthcare professionals.
©2022 Sanofi. All rights reserved. MAT-GLB-2203038 v1 September 2022.
Sanofi and Regeneron are committed to providing resources to advance knowledge in areas of unmet medical need among patients with inflammatory and immunologic diseases.