Managing CSU
Improve your ability to recognise and manage chronic spontaneous urticaria (CSU).
- Explore engaging video content for expert insights into current therapeutic options
- Delve into real-world efficacy data on treatments
- Familiarise yourself with the pathophysiology and symptoms of CSU
- Review national and international guidelines
Developed by EPG Health for Medthority. This content has been developed independently of the sponsor Novartis Pharma AG
Prevalence of CSU
Urticaria is more common than previously thought1
Though there is little epidemiological data for chronic urticaria (CU), a recent meta-analysis reported a point and lifetime prevalence rates of 0.7% and 1.4% respectively1. Interestingly, the results of this study also suggest that CU prevalence is on the rise, though there is substantial variation between geographical regions1.
Overall, chronic spontaneous urticaria (CSU) accounts for over two-thirds of the cases of CU, and most studies show that it is more common among women than men1,2.
Terminology
Lifetime prevalence is the proportion of a population that at some point in their life (up to the time of the assessment) have experienced the condition3
Figure 1. CSU as a percentage of chronic urticaria cases and the ratio of male to female CSU sufferers (Adapted2,4). CIndU, chronic inducible urticaria; CSU, chronic spontaneous urticaria.
Although all age groups can be affected, the peak incidence is seen between 20 and 40 years of age5. There is no apparent correlation of prevalence with education, income, occupation, place of residence or ethnic background; although prevalence of chronic urticaria has been noted as higher in Asian studies compared with Europe or the USA1. Interestingly, a Korean study published in 2017 also found that CSU had a greater impact on children and the elderly than was expected6.
The majority of patients have no evidence of any exacerbating factor, however a recognised trigger for CSU is nonsteroidal anti-inflammatory drugs7.
A population-based study on the epidemiology of CSU in Italy over a 12-year period (2002–2013) demonstrated that the risk of CSU was statistically significantly higher in the presence of the following variables8:
- obesity
- anxiety
- dissociative and somatoform disorders
- malignancies
- use of immunosuppressive drug
- schronic use of systemic corticosteroids
CSU duration
CSU has a duration of at least 1 year in most patients and more than 5 years in a considerable proportion. In very rare cases it can last up to 50 years5,9–12.
Figure 2. The percentage of patients with CSU symptoms at 6 months, 3 years, 5 years, and 25 years after their initial diagnosis (Adapted13).
The evolution of CSU is unpredictable, with spontaneous remissions and relapses7. At least 50% of CSU patients will experience at least one recurrence of CSU symptoms after an apparent resolution14
Prognostic factors for the duration of CSU
The duration of CSU is generally longer in patients with:
- more severe disease5,9
- concurrent angioedema5,9
- concurrent inducible urticaria5,15
- a positive autologous serum skin test (ASST) or autologous plasma skin test (APST)5,16,17
The autologous serum skin test (ASST) is a screening test for autoantibodies in CSU.
Diagnosis is based on a thorough medical history and physical examination as well as diagnostic tests18.
Acute vs. chronic urticaria
While the lifetime prevalence for CSU estimates range from 0.6% to 1.8%19,20, the lifetime prevalence for acute urticaria is thought to be as high as 20%18. Furthermore, acute urticaria accounts for 7–35% of dermatological conditions seen in emergency care21.
Acute urticaria is defined as the occurrence of spontaneous hives, angioedema, or both for <6 weeks18.
Acute urticaria aetiology
The underlying causes of acute urticaria remain idiopathic in 50% of cases, with infections responsible for approximately 40%, and adverse reactions to drugs (9%) and food (1%) responsible for the remaining 10% of cases22.
Managing acute urticaria
For patients with an identifiable trigger, for example a food allergy, patients should be investigated to confirm sensitisation enabling avoidance of the trigger and prevention of future episodes. With acute urticaria being time-limited, treatment is usually focused on symptomatic relief with second-generation H1-antihistamines18. In a study of 100 acute urticaria patients presenting at a French emergency department, 79% of patients treated with levocetirizine were itch free after two days21.
Current guidelines also state that a short course of oral corticosteroids (up to 10 days) may help reduce the duration and activity of acute exacerbations of urticaria18. Interestingly, in the above study, addition of prednisone to levocetirizine treatment did not improve the symptomatic or clinical response compared with levocetirizine plus placebo. This suggests that the addition of a corticosteroid to antihistamine therapy may be unnecessary in acute urticaria patients21.
Sensitisation to insect bites, such as mosquitos, is common and results in immediate hives and pruritic bite papules, although systemic anaphylactic reactions can also occur23. Mosquito-bite hives are a result of antisaliva IgE antibodies and histamine release meaning oral second-generation H1-antihistamines can be an effective option for management of the hives and itch. Placebo-controlled trials have shown cetirizine, ebastine and rupatadine to be effective treatment options in mosquito-bite allergic adult patients. Prophylactically administered rupatadine 10 mg resulted in a 48% decrease in mean hive size and a 21% reduction in itch in these patients. Importantly, for a condition characterised by intense pruritus, rupatadine 10 mg was observed to have a rapid onset of action with a significant reduction versus placebo in hive size and itch reported 15 minutes after administration23. In a separate study, prophylactic cetirizine 10 mg and ebastine 10 mg, but not loratadine 10 mg, resulted in a significant reduction in hive size. While cetirizine had a significantly greater effect on itch than ebastine and loratadine, it also increased the levels of sedation observed, although the clinical significance was uncertain as no patients dropped out23.
Burden of disease
The impact on patients’ lives goes beyond effects on skin; currently treated patients experience higher levels of health-related impairment in their functioning in work and non-work activities and quality of life and are more frequent users of healthcare than similar individuals without the condition24
Quality of life
CSU adversely affects many aspects of patients’ lives5
Figure 3. The impacts of CSU on quality of life (Adapted25,26).
Many aspects of quality of life (QoL) are found to be reduced in patients with CSU27 and the presence of angioedema further impairs QoL scores28. The QoL of patients with CSU is at least as impaired as in patients with other skin diseases, with higher impact on daily living and physical discomfort29.
Measurement of patient QoL
QoL in CSU patients may be measured using specific skin disease questionnaires such as the Dermatology Quality of Life Index (DQLI)30,31
Factors contributing to a reduced QoL
In addition to the classical symptoms associated with CSU, factors of major importance to patients that contribute to a reduced QoL include11:
- unpredictability of attacks
- persistent lack of sleep
- fatigue
- disfigurement
Patients with CSU also often have comorbidities such as depression and anxiety32. Over 60% of CSU patients report anxiety at some point during their flares11 and 17% are diagnosed with depressive or somatoform disorders33.
Disease that is refractory to antihistamines is also potentially a much larger problem than previously believed. In 2019, 1-year data from the AWARE study was published34. This suggested that antihistamine-refractory patients demonstrate high levels of uncontrolled disease, angioedema and co-morbid chronic inducible urticaria (CIndU). They were noted to have impaired QoL, be generally undertreated, and are increasingly dependent on healthcare services35,36; most patients did not receive guideline-recommended treatments35. The Scandinavian arm of the AWARE study noted high rates of healthcare utilisation and QoL impairment37.
The RELEASE survey study in Japan evaluated real world QoL impairment, reporting similar findings to the AWARE study. Findings suggest that patients with worse urticaria have greater work productivity loss and activity impairment compared with milder cases, and report greater dissatisfaction with their health and treatment38.
Visit Industry-Sponsored AWARE study eLearning module
The socioeconomic burden
The socioeconomic cost of CSU is high in terms of direct medical costs and indirect costs, such as lost wages because of absences from work5,39. It is estimated that approximately 60–70% of patients report absence from work or school as a direct result of their CSU11,39 and 26% report that it causes three or more days’ absence a year39. Patients with severe CSU incur higher direct and indirect costs than patients with mild or moderate disease. Most patients, particularly those with angioedema, will need continuous medication to alleviate symptoms and regular visits to healthcare facilities5,39.
Almost three-quarters of patients with moderate to severe CSU attend a healthcare professional at least once per year (mean of 3.3); consultant allergists (39.0%) and dermatologists (38.5%) are the most common specialties visited40.
In addition, CSU is heterogeneous in its regional management, with healthcare utilisation and outcomes differing between healthcare systems. However, there is still a high overall unmet need in CSU patients associated with healthcare resources use, and a large negative effect on QoL and work productivity41.
CSU pathophysiology
Chronic spontaneous urticaria (CSU) is driven by the activation of mast cells, which release histamines and other immune modulators, although the precise mechanism is not fully known42.
Mast cell activation in CSU
Urticaria is a mast-cell-driven disease18.
Figure 4. Interactions of mast cells on urticaria symptoms (Adapted43,44).
Though the pathophysiology of urticaria is complex and yet to be fully characterised, it is thought that activated mast cells release histamine and other inflammatory mediators, such as platelet-activating factor (PAF) and cytokines45. The mediators cause sensory nerve activation, vasodilatation, and plasma extravasation as well as cell recruitment to urticarial lesions, and form the basis for antihistamines being first-line management12,46,47.
CSU skin lesions show recruitment of mast cells, basophils, neutrophils, eosinophils and T-lymphocytes18,48–52.
Figure 5. The recruitment of mast cells, basophils, neutrophils, eosinophils and T-lymphocytes in CSU skin lesions (Adapted18,48–52).
The mast cell activating signals in urticaria are ill-defined and likely to be heterogeneous and diverse18.
Basophils
Basophils, along with mast cells, play an important role in the pathophysiology of CSU. Peripheral blood basophils from CSU patients have unique features that reverse upon remission and in response to therapy7,53:
- basopaenia is typically found in CSU patients
- basophils from CSU patients also tend be less responsive to stimuli that act through the IgE receptor
- basophils from CSU patients are hyperresponsive when stimulated with other sera regardless of source
Immunoglobulin E
Immunoglobulin E (IgE) is key to the release of histamine and other pro-inflammatory mediators from mast cells and basophils and may play a role in the pathogenesis of CSU18.
IgE binds to high-affinity (FcεRI) receptors on mast cells, basophils, eosinophils, alveolar macrophages and antigen-presenting cells54–56. Cross-linking of IgE bound to FcεRI receptors triggers degranulation and release of inflammatory mediators55–57. There is a strong association between IgE and allergic conditions57.
The FcεRI receptor on mast cells plays a key role in activation of these cells and in the pathophysiology of CSU58,59.
Figure 6. IgE interactions with mast cells in CSU (Adapted42,60–62). IgE, immunoglobulin E; PAF, platelet-activating factor.
Mast cell activation may either be via autoimmune, allergic or idiopathic mechanisms61–64. It is generally believed that allergy is not an underlying cause of CSU, although total IgE levels are typically higher in CSU patients than in healthy individuals62,65.
CSU Pathogenesis
A summary of the current understanding of the pathogenesis of CSU is illustrated in Figure 7.
Figure 7. Pathogenesis of CSU summary. CSU, chronic spontaneous urticaria; IgE, immunoglobulin E; IgG, immunoglobulin G; PAF, platelet-activating factor; TPO, thyroid peroxidase (Adapted64,66,67).
Potential role of histamine intolerance in CSU
It has been suggested that chronic infections, autoreactivity and intolerance to food may play a role in CSU. The type of food intolerance described differs from regular IgE-mediated food allergy as it involves a pseudoallergenic response to artificial additives, natural compounds and dietary histamines. A study was conducted by Siebenhaar et al. in 2016 with the aim of determining the rate that this histamine intolerance was evident in patients with CSU68.
It was noted that from history alone it was impossible to determine whether a pseudoallergen-free diet would help the symptoms of CSU sufferers, however, avoidance diets did improve the symptoms of some urticaria sufferers. However, it was reported that histamine intolerance was a rare comorbidity68.
CSU symptoms
The symptoms of chronic spontaneous urticaria (CSU) include itchy hives (wheals) and angioedema18
The symptoms of CSU may appear without warning with a variable intensity5,18 and may profoundly impact patients' day-to-day lives5,27,32,69,70. In CSU, itchy hives, angioedema or both, may occur spontaneously every day, or almost daily for six weeks or more18.
Hives
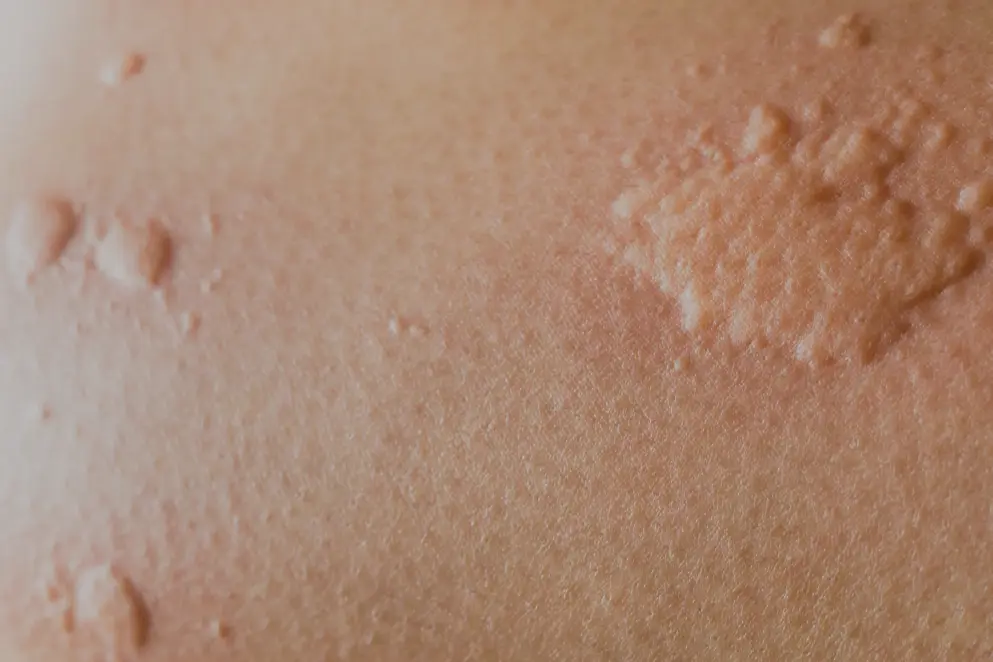
A hive consists of three typical features18:
- Central swelling of variable size, usually surrounded by a reflex erythema (Figure 8)
- Associated itching (pruritus), or sometimes a burning sensation
- Transient nature, usually resolving within 30 minutes to 24 hours
Figure 8. Hives are superficial swellings with pale centres surrounded by a red flare71 .
Terminology
The terms ‘itch’ and ‘pruritus’ are interchangeable, as are ‘hive’ and ‘wheal’
Reflex erythema describes the redness of the skin due to dilated capillaries that is triggered by local neural reflexes. Erythema typically blanches with pressure
Angioedema
Angioedema (deep tissue swelling) is typically characterised by18:
- sudden, pronounced swelling or redness of the lower dermis and subcutis or mucous membranes (hypodermis or superficial fascia)
- sometimes pain rather than itching
- up to 72 hours for resolution
The eyelids and lips are most commonly affected, while the tongue, extremities, genitalia, oral cavity mucosa and upper respiratory tract may also be affected72.
Figure 9. Percentage of CSU patients with angioedema alone, hives or both (Adapted73).
Relationship between CSU symptoms and quality of life
In CSU, changes in symptom severity are closely linked to changes in health-related quality of life (HRQoL). If an improvement (or worsening) in signs and symptoms is found, it is highly likely that an improvement (or worsening) HRQoL is also experienced74
CSU diagnosis and assessment
Since there is no definitive test for chronic spontaneous urticaria, diagnosis is based on a thorough medical history and physical examination as well as diagnostic tests18.
The importance of earlier diagnosis of CSU
Despite the clear diagnostic algorithm included in international guidelines, evidence suggests that many patients with CSU experience delays in receiving a diagnosis18,40,75,76
The Assessment of the Economic and Humanistic Burden of Chronic Spontaneous/Idiopathic Urticaria Patients (ASSURE-CSU) aimed to provide real-world evidence on the unmet needs of patients with uncontrolled CSU by assessing various aspects of the patient experience. Among the 673 patients included in the study, the mean disease duration was 4.8 years, and the mean (SD) duration of disease from symptom onset to diagnosis was 24.0 (63.36) months, with a median delay of 4.7 months. The authors attributed this delay in diagnosis and specialist referral to a lack of specialist knowledge in the primary and secondary care settings with regard to CSU40.
For patients, these delays in obtaining a diagnosis represent a source of great frustration. Many of them will visit multiple healthcare providers before their diagnosis is confirmed and appropriate treatment is initiated75. For example, in an Italian study published in 2017, three-quarters of the 190 patients included visited ≥3 different physicians before finally receiving a diagnosis of CSU76. Other studies have shown that during this process, patients frequently resort to online resources in search of answers75. This may result in inaccurate self-diagnoses and, alarmingly, attempts to self-medicate are not uncommon. There have even been reports of patients using unprescribed prednisone in a bid to alleviate their symptoms75.
Of the patients included in the Italian study described above, 57% expressed hope for a more rapid and straightforward treatment process, particularly in terms of diagnosis76. Better education for both patients and physicians to help raise awareness of CSU could help spare patients the frustration and anxiety associated with delayed diagnosis and facilitate earlier interventions with appropriate therapeutic agents75.
Find out more about the journey to diagnosis from the patient perspective with our podcast, “All Things Urticaria: The Patient Voice”.
CSU diagnosis
Awareness and understanding of CSU are important to ensure correct diagnosis and appropriate treatment or referral18. Guidelines for diagnosis recommend a thorough patient history and physical examination followed by routine diagnostic tests18,77. Extended diagnostic tests may be needed, based on patient history, to exclude differential diagnoses.
The 2017 International EAACI/GA2LEN/EDF/WAO guidelines recommend a three-step process for the effective diagnosis of urticaria (Figure 10)18.
Figure 10. The three steps recommended in the 2017 International EAACI/GA2LEN/EDF/WAO guidelines for the effective diagnosis of urticaria (Adapted18). EAACI, European Academy of Allergy and Clinical Immunology; EDF, European Dermatology Forum; GA2LEN, Global Allergy and Asthma European Network; WAO, World Allergy Organization.
Patient history in CSU
Step 1
The first step in the diagnosis of urticaria should be to take a thorough patient history18
Recommended questions should take into consideration a number of different factors including18,77:
- duration of disease
- physical symptoms
- provoking factors
- family history
- impact on everyday life
- previous diagnostic tests/therapy
Duration of disease
- Time of onset
- Frequency/duration and provoking factors for hives
- Diurnal variation
- Occurrence in relation to weekends, holidays and/or foreign travel
Physical symptoms of CSU
- Shape, size and distribution of hives
- Associated angioedema
- Associated symptoms (e.g. bone/joint pain, fever abdominal pain)
See also Assessment Tools for disease activity and impact in CSU
CSU provoking factors
- Induction by physical agents or exercise
- Observed correlation to food
Family history in CSU
- Family and personal history regarding wheals and angioedema
- Previous or current allergies
- Gastric/intestinal problems
- Association with infections
- Use of alternative therapeutic drugs (e.g. nonsteroidal anti-inflammatory drugs [NSAIDs], angiotensin-converting-enzyme [ACE]-inhibitors)
- Relationship to the menstrual cycle
- Smoking habits (especially use of perfumed tobacco products or cannabis)
- Type of work, hobbies
- Stress (eustress and distress)
- Occurrence in relation to travel
Impact of CSU on everyday life
- Quality of life (QoL) related to urticaria and emotional impact
See also Assessment Tools for disease activity and impact in CSU
Previous diagnostic tests/therapy in CSU
- Previous therapy and response to therapy
- Previous diagnostic procedures/results
Figure 11. Examples of the type of questions that may be asked when taking a clinical history (Adapted77). ACE, angiotensin-converting enzyme; NSAID, non-steroidal anti-inflammatory drugs.
Physical examination in CSU
Step 2
The second step in the diagnosis of urticaria should be to conduct a physical examination18
Physical examination should include:
- identification and characterisation of any current lesions
- diagnostic provocation tests if indicated if chronic inducible urticaria (CIndU) is suspected
- checking for signs of systemic illness
Table 1. Diagnostic provocation tests for CIndU (Adapted78).
Diagnostic tests for CSU
Step 3
As a third step, diagnostic tests should be performed as appropriate18
Routine diagnostic tests
Only very limited routine diagnostic tests should be performed in CSU and these tests should not be performed in acute urticaria18.
Figure 12. Routine diagnostic tests in CSU (Adapted18). CRP, C-reactive protein; CSU, chronic spontaneous urticaria; ESR, erythrocyte sedimentation rate.
Extended diagnostic tests
Extended diagnostic tests may be needed, based on patient history, to exclude differential diagnoses18.
Extended diagnostic tests may be considered where directed by patient history, for identification of underlying causes and for ruling out possible differential diagnoses if indicated (Figure 13). Unless strongly indicated by patient history (e.g., allergy) extended diagnostic tests should not be carried out in acute spontaneous urticaria18.
Figure 13. Extended diagnostic tests in CSU (Adapted18). ASST, autologous serum skin test; CSU, chronic spontaneous urticaria.
Infectious diseases
- Bacterial, viral, parasitic or fungal infections have been implicated as underlying causes of CSU79
- e.g., H. pylori, Streptococci, Staphylococci, Versinia, Giardia lamblia, Mycoplasma pneumonia, Hepatitis virus, Norovirus, Parvovirus B19, Anisakis simplex, Entamoeba, Blastocystis
- The frequency and relevance of infectious diseases varies between different patient groups and geographical regions18,79
- More research is needed to make definitive recommendations regarding the role of infection in urticaria18,79
Type I allergy or pseudo-allergic reactions
- Type I allergy is a rare cause of CSU in patients who present with daily or almost daily symptoms, but may be considered in CSU patients with intermittent symptoms79,80
- Pseudo-allergic (non-allergic hypersensitivity) reactions to NSAIDs, food, or food additives may be more relevant for CSU with daily symptoms79
- Diagnosis should be based on an easy-to-follow diet protocol79
The autologous serum skin test (ASST)
A positive ASST is indicative of CSU42,81. The presence of autoantibodies can aid in the diagnosis of CSU. The ASST is the only test available to screen for autoantibodies in CSU, against either immunoglobulin E (IgE) or high-affinity (FcεRI) receptors81. Confirmation of functional autoantibodies requires a Basophil Histamine Release Assay (BHRA)81,82.
The ASST:
- assesses autoreactivity in response to serum collected during active disease77
- evaluates the presence of histamine-releasing factors (including autoantibodies)42,81
A positive ASST response is defined by an inflammatory red wheal response81.
Lesional skin biopsy
- If urticarial vasculitis is suspected, biopsy of lesional skin should be carried out18
- Damage of the small vessels in the papillary and reticular dermis and/or fibrinoid deposits in perivascular and interstitial locations are suggestive of UV (urticarial vasculitis)18
Differential diagnosis
Recurrent hives and angioedema may occur in various different diseases (Figure 14), so excluding differential diagnoses is an important aspect of the diagnostic work up for CSU18.
Figure 14. Differential diagnoses for patients with hives and angioedema (Adapted18). AAE, acquired angioedema; ACE, angiotensin converting enzyme; AID, acquired autoinflammatory disorders; HAE, hereditary angioedema; IgD, hyperimmunoglobulinemia D; TNF, tumour necrosis factor.
Recommended diagnostic algorithm for urticaria
Figure 15. Diagnostic algorithm for CSU (Adapted18). AAE, acquired angioedema; ACE, angiotensin converting enzyme; AE, angioedema; AID, acquired autoinflammatory disorders; HAE, hereditary angioedema.
Assessment tools
Assessment tools for disease activity and impact in CSU
International urticaria guidelines recommend the use of tools in CSU patients to measure and monitor disease activity and health-related quality of life (HRQoL)18. Physicians have a choice of tools (generic or CSU specific) to assess the severity impact of CSU and treatment response. In CSU, changes in symptom severity are closely linked to changes in HRQoL74.
Table 2. Disease activity and impact tools (Adapted10,18,30,83–85).
| Measurement | Hives and Itch | Angioedema |
| Disease activity | · Urticaria Activity Score (UAS) · Weekly Urticaria Activity Score (UAS7) |
· Angioedema Activity Score (AAS) |
| Urticaria-specific QoL | · Chronic Urticaria · Quality of Life Questionnaire (CU-Q2oL) |
· Angioedema · Quality of Life Questionnaire (AE-QoL) |
| Dermatology-specific QoL | · Dermatology Life Quality Index (DLQI) | |
| Disease activity, disease-specific QoL and treatment effectiveness | · Urticaria Control Test (UCT) | |
The Urticaria Activity Score (UAS)
The UAS is a validated daily measure, encompassing hives and itch, to assess urticaria severity and monitor treatment outcomes (Table 3)18,31.
Table 3. Urticaria activity score (Adapted18). ISS, Itch Severity Score.
The daily UAS (range, 0–6) equals the sum of daily Itch Severity Score (ISS) and the hives score18,86.
The UAS7 (range, 0–42) is a weekly composite of the daily UAS (Figure 16)86.
Figure 16. Calculating the UAS7 (Adapted18,86). UAS7, 7-day Urticaria Activity Score.
UAS7 is a measure of CSU severity over 7 days5,18 (Figure 17).
Figure 17. Interpreting the UAS7 (Adapted18,84). UAS7, 7-day Urticaria Activity Score.
A variation of the UAS7, the UAS7TD was assessed to determine whether the UAS7 could be used to divide patients into subgroups to better assess impact on quality of life87. It was found that categorical UAS7 subgroups could be used to predict differences among patients with different levels of disease severity – this may enable better clinical monitoring of treatment efficacy87.
The Angioedema Activity Score (AAS)
The AAS is used to assess disease activity in patients with recurrent angioedema18.
The AAS allows the patient to score each of five key factors relating to their symptoms from 0 to 3 (giving a daily score of 0–15). Daily AAS can be summed to give 7-day scores (AAS7), 4-week scores (AAS28), and 12-week scores (AAS84)88.
The Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL)
The CU-Q2oL patient questionnaire was designed and validated for the assessment of QoL specifically in chronic urticaria, including the physical, psychosocial and practical aspects of this condition89. It consists of 23 questions covering six key domains relevant to89:
- pruritus
- swelling
- impact on life activities
- sleep problems
- looks
The effects of disease on each domain are scored from 1 (not at all) to 5 (extremely) and totalled to give an overall score ranging from 0–92 with higher scores indicating a greater impairment in QoL. The CU-Q2oL is simple to administer and requires approximately five minutes to complete89.
The Angioedema Quality-of-Life Questionnaire (AE-QoL)
The AE-QoL is the first angioedema-specific patient-reported QoL questionnaire. It consists of 17 questions across 4 domains (Figure 18)85.
Figure 18. AE-QoL Questionnaire Domains (Adapted85).
The Dermatology Life Quality Index (DLQI)
DLQI is a validated and widely used dermatology-specific patient questionnaire for evaluating HR-QoL in patients with a variety of skin conditions including CSU30,31. It is used in clinical practice and trials but was not included in the last international urticaria guidelines18.
The Urticaria Control Test (UCT)
The UCT is a single questionnaire comprised of four questions designed to evaluate the physical symptoms of chronic urticaria (itch, hives and/or angioedema), the impact on HR-QoL and the effectiveness of treatment over four weeks85. It consists of just four simple questions allowing rapid and easy completion and is valid for both CSU and CIndU85,90.
Fast facts
Understanding Chronic Spontaneous Urticaria (CSU) and its impact is crucial to optimise treatment outcomes. Here are some key points to help better understand this debilitating skin condition18,33,69,83,89,91–93:
Comorbidities in CSU
Unfortunately for many patients with chronic spontaneous urticaria (CSU), the itchy hives and/or angioedema associated with the condition is not all they have to contend with. A substantial number of patients also experience comorbidities associated with the development of CSU94.
Autoimmune diseases in CSU
The pathogenesis of CSU in a subset of patients is believed to be a consequence of an autoimmune response driven by immunoglobulin E (IgE) or immunoglobulin G (IgG) autoantibodies94,95, and a strong association is found between CSU and major autoimmune diseases96, with evidence suggesting potential autoimmune aetiology in up to 50% of CSU patients42. Patients with CSU, therefore, are thought to be at an increased risk of developing other autoimmune disorders. In fact, while the global prevalence of autoimmune diseases is considered to be ≤1%, in patients with CSU it is thought to be ≥1%94,95.
Evidence suggests a potential autoimmune aetiology in up to 50% of patients with CSU42
A large Israeli population study exploring the links between CSU and other autoimmune conditions found that a diagnosis of CU was associated with an increased risk of developing hypothyroidism (9.8% vs. 0.6%; p<0.0005) and hyperthyroidism (2.6% vs. 0.09%; p<0.0005). Interestingly, the presence of autoimmune disease was significantly higher in female patients than male patients. A similar effect was seen with type 1 diabetes (female patients OR, 12.92; 95% CI, 6.53–25.53; p<0.0005 vs. male patients OR, 2.34; 95% CI, 1.15–4.73; p=0.01). Importantly, the onset of type 1 diabetes was observed in the majority (84.8%) of patients in the years after receiving a diagnosis of CU. Meanwhile, a number of autoimmune conditions were only significantly increased in female patients. These included rheumatoid arthritis (OR, 19.88; 95% CI, 10.15–38.92; p<0.0005), Sjögen syndrome (OR, 23.30; 95% CI, 7.31–74.20; p<0.0005), coeliac disease (OR, 57.83; 95% CI, 7.99–418.29; p<0.0005) and systemic lupus erythematosus (OR, 26.71; 95% CI, 6.49–109.90; p<0.0005)96.
More recently, a Korean study utilised their national database to explore the presence of various conditions in patients with CU, patients with CSU and patients without CU/CSU. Similar to Confino-Cohen et al., Korean patients with CU (12.34%) or CSU (11.34%) had a significantly increased rate of autoimmune thyroid diseases (AITD) compared to controls (5.49%)6.
Alopecia areata (AA) is another autoimmune disease with a global prevalence of 0.1–0.2%. However, this differs between populations and studies with an observed prevalence of ~0.7–3% in the USA, and ~2% in the UK. An Israeli study matched 1,751 patients with AA to 3,502 control patients and assessed their respective comorbidities. Interestingly, patients with AA had a significantly increased risk of having comorbid CSU (OR, 6.15; 95% CI, 4.06–9.32; p<0.001) than the control group. Furthermore, patients with both AA and CSU were more likely to also have comorbid allergic rhinitis and atopic dermatitis than patients with CSU but not AA in the control group97.
To gain greater clarity on the association of AITD and CSU, a systematic literature review compared data from 169 identified publications. A strong correlation was seen between CSU and elevated IgG antithyroid autoantibodies, in particular IgG-anti-TPO antibodies. Furthermore, some evidence suggests that patients with CSU typically have higher levels of IgE-anti-TPO autoantibodies than controls. As expected, these changes in autoantibody levels are also associated with elevated rates of AITD. It was identified that patients with CSU are more likely to experience hypothyroidism and Hashimoto’s thyroiditis than hyperthyroidism and Grave’s disease. In addition, and supporting the Israeli population study, thyroid dysfunction was more commonly observed in female than male patients with CSU94,96.
A separate systematic literature review looked at the published rates of a broader spectrum of autoimmune diseases in patients with CSU. The rates of comorbidity in the majority of studies were ≥1% for insulin-dependent diabetes mellitus, rheumatoid arthritis, psoriasis and coeliac disease, ≥2% for Grave’s disease, ≥3% for vitiligo and ≥5% for pernicious anaemia and Hashimoto’s thyroiditis95.
To find out more about autoimmune comorbidities in CSU, check out our All Things Urticaria podcast episode ‘Let’s talk comorbidities’ with Professor Marcus Maurer and Dr Simon Francis Thomsen.
Allergic disease
While a link between chronic urticaria and atopic diseases has been suggested, until recently the epidemiological data was lacking98, but some data are starting to clarify the situation. Among a Korean population of patients with CU or CSU, the likelihood or having comorbid allergic rhinitis, drug or other allergies, or asthma was approximately 4.68 times higher than in the control group (Table 4)6.
Table 4. Mean percentage of patients diagnosed with comorbidity between 2010 and 2013 in Korea (Adapted6).CSU, chronic spontaneous urticaria; CU, chronic urticaria.
In an Israeli population study, 11,271 patients with CU were compared to 67,216 age- and sex-matched controls98. Interestingly, while fewer people experienced allergic comorbidities than observed in the Korean study, they were still significantly more common in patients with CU than in the control group. In this setting, 10.8%, 9.8% and 19.9% of patients with CU had been diagnosed with asthma, atopic dermatitis or allergic rhinitis, respectively compared to 6.5%, 3.7% and 10.1% of patients in the control group98. A multivariate analysis that adjusted for age, sex, body mass index, smoking status and ethnicity revealed that CU was significantly associated allergic rhinitis (OR, 2.03; p<0.001), atopic dermatitis (OR, 2.77; p<0.001) and asthma (OR, 1.62; p<0.001)98. Meanwhile, a comparison of elderly (>60 years of age) and non-elderly patients with CU in Korea revealed that elderly patients with CU were significantly more likely to have comorbid atopic dermatitis than non-elderly patients (37.8% vs. 21.7%, p=0.022)99. However, no difference was seen in the prevalence of asthma or allergic rhinitis99.
The role of allergies and mast cells in irritable bowel syndrome (IBS) has gained increased attention recently. With this possible pathophysiological similarity between IBS and CU, Shalom et al. produced a follow-up study addressing the epidemiological links between the two conditions in Israel98. A total of 1.7% of patients with CU had concomitant IBS versus 0.8% of controls (p<0.001) giving an OR of 1.86 (95% CI, 1.57–2.19; p<0.001). While a pathophysiological explanation remains hypothetical, this study does suggest an association between IBS and CU, warranting further investigation98.
Psychiatric conditions in CSU
Dermatological conditions can have a substantial impact on the mental wellbeing of patients. A UK study reported that 17% of dermatology patients required psychological support while 85% reported that the psychosocial aspects of their skin condition are a major component of their illness100. More recently, a systematic review and meta-analysis including a total of 25 studies revealed that nearly one third of patients with chronic urticaria have one or more psychiatric comorbidity101.
It has been reported that nearly one third of patients with CU have at least one underlying psychiatric comorbidity101
The psychological burden of CSU is significant. In a survey of 369 patients with CSU, the prevalence of psychological issues was roughly twice as high as reported by matched controls (Figure 19)24.
Figure 19. Prevalence of mental health comorbidities and sleep difficulties among patients with CSU and matched controls (Adapted24). CSU, chronic spontaneous urticaria.
Similar results were reported in a German study of 100 patients with CSU who were screened for mental health comorbidities. Among this group of patients, 48% had one or more mental health disorder with the most common being anxiety (30%) and depressive and somatoform disorders (17% each)33.
The psychological comorbidities associated with CSU have been shown to extend beyond anxiety and depression. Patients with CSU also have higher levels of alexithymia (the inability to identify and communicate emotions) than healthy controls and may be related to a link between pruritus severity and state anger as assessed by the State-Trait Anger Inventory (STAXI)102. Furthermore, post-traumatic stress disorder (PTSD) has been associated with CSU with 34% of patients with CSU in one study meeting the diagnostic criteria for PTSD vs. 18% of allergy control patients103–105.
The impact of these associated comorbidities should not be underestimated. In a study of 746 patients with CU and 5,107 patients with psoriasis, patients with CU had a comparable impairment of mental/physical health to those patients with moderate-to-severe psoriasis (Figure 20)106.
Figure 20. Psychological burden of CU and psoriasis (Adapted106). CU, chronic urticaria.
To find out more about psychiatric comorbidities in CSU, check out our All Things Urticaria podcast episode ‘Let’s talk comorbidities’ with Professor Marcus Maurer and Dr Simon Francis Thomsen.
The Aim of Treatment
The aim of treatment for urticaria is quick and complete symptom control5,18,107
The recommended treatment algorithm
The 2017 European Academy of Allergy and Clinical Immunology (EAACI)/Global Allergy and Asthma European Network (GA2LEN)/European Dermatology Forum (EDF)/World Allergy Organization (WAO) guidelines recommend the following step-wise approach to the treatment of urticaria (Figure 21)18.
Figure 21. The 2017 EAACI/GA2LEN/EDF/WAO recommended treatment algorithm for chronic urticaria (Adapted18). EAACI, European Academy of Allergy and Clinical Immunology; EDF, European Dermatology Forum; GA2LEN, Global Allergy and Asthma European Network; WAO, World Allergy Organisation.
A number of additional treatment options are mentioned in the EAACI/GA2LEN/EDF/WAO guidelines but are not included in the recommended treatment algorithm due to limited supporting evidence18.
It is recommended to reassess the need for continued or alternative drug treatment every three to six months, as the severity and symptoms of urticaria may fluctuate and spontaneous remission may occur at any time18.
An urticaria treatment algorithm should serve both patients with easy-to-treat symptoms and those more refractory to treatment and should allow the stepping up or stepping down of treatment depending on requirements over time108.
First-line therapy for CSU
International EAACI/GA2LEN/EDF/WAO guidelines recommend the use of second generation H1-antihistamines, at licensed doses, as first-line treatment for CSU18.
2017 updates to the international CSU guidelines
First generation H1-antihistamines are no longer recommended for the treatment of urticaria due to5,109,110:
- pronounced central nervous system (CNS) and anticholinergic effects/interference with rapid eye movement (REM) sleep (sedating effects)
- drug interactions, particularly with drugs affecting the CNS, e.g., analgesics, hypnotics, sedatives, mood elevating drugs and alcohol
Figure 22. Potential adverse effects of first (old)-generation H1-antihistamines (Adapted111). CNS, central nervous system.
Second generation H1-antihistamines are well tolerated by most patients and are non-sedating or minimally sedating, and free from anticholinergic effects18. However, astemizole and terfenadine, two of the earlier modern second-generation drugs, require hepatic metabolism for full activation and have cardiotoxic effects if this metabolism is blocked18. Due to these safety concerns, these two drugs are no longer available in most countries, and are not recommended in the CSU guidelines18. Despite this setback, newer modern second-generation antihistamines were developed to overcome these issues. Initially, the new generation of antihistamines comprised cetirizine (metabolite of hydroxyzine), loratadine, and fexofenadine but now includes acrivastine, azelastine, bepotastine, bilastine, desloratadine, ebastine, epinastine, levocetirizine, mequitanzine, mizolastine, olopatadine, and rupatadine18.
While a total of 16 different second generation H1-antihistamines have featured in review articles and clinical studies focussed on the treatment of CSU, only 10 are widely recognised in current guidelines (acrivastine, bilastine, cetirizine/levocetirizine, ebastine, fexofenadine, loratadine/desloratadine, mizolastine, and rupatadine)112.
Why not combine first- and second-generation H1-antihistamines?
While second-generation antihistamines are the recommended first-line therapy in CSU, many physicians continue to believe that the addition of a sedating, first-generation antihistamine in the evening can aid patient sleep. However, the results from a randomised, double-blind, cross-over study indicated that the addition of hydroxyzine to a second-generation antihistamine did not improve patient sleep, but did increase daytime somnolence, supporting the use of second-generation antihistamines only in the treatment of CSU110,113.
Clinical evidence
Several second-generation H1-antihistamines are used for the management of CSU. Here we review the available clinical data in adults for each.
Bilastine
Bilastine is a second-generation H1-antihistamine that is used for the management of allergic rhinitis and urticaria. A double-blind, randomised, placebo-controlled trial evaluated the efficacy and safety of 20 mg bilastine versus 5 mg levocetirizine and placebo in adult patients with CSU114. Over a 4-week period, 525 patients were assessed with total symptom scores used as the primary endpoint. From day 2 onwards, bilastine showed a significant improvement in the patients’ symptoms compared with placebo. Furthermore, bilastine treatment also resulted in significant improvements compared with placebo in Dermatology Life Quality Index (DLQI) scores, as well urticaria-associated discomfort, and sleep disruption. When considering the levocetirizine group, bilastine was observed to have comparable efficacy and tolerability114. A Japanese study has indicated that the efficacy and tolerability of bilastine is maintained over the course of a year115.
Cetirizine
Clinical data on the efficacy and safety of cetirizine in patients with CSU has been available for 30 years. In 1988, 30 patients with CSU were treated with 10 mg cetirizine or placebo in a double-blind cross-over trial116. This early data indicated that cetirizine significantly reduced the occurrence of hives and pruritus compared with placebo (p<0.001). Of the 30 patients, 26 improved on cetirizine, two on placebo and two discontinued cetirizine due to a lack of efficacy. Meanwhile, mild sedation was observed in two patients receiving cetirizine and one patient given placebo116.
Head-to-head
Cetirizine has also been evaluated in CSU in several head-to-head trials. An early study compared cetirizine with the first-generation antihistamine hydroxyzine and placebo117. While cetirizine was shown to have similar efficacy to hydroxyzine, it had a lower incidence of somnolence and showed levels not significantly different to those observed in the placebo group117.
More recently, the effectiveness and safety of cetirizine was compared to that of rupatadine. In a randomised, double-blind, 6-week trial, 70 patients with CSU were treated with 10 mg of cetirizine or rupatadine once daily118. Evaluations of the mean number of hives (wheals), mean pruritus score and mean total symptom score revealed significantly greater improvements with rupatadine than cetirizine (Figure 23)118.
Figure 23. Mean change from baseline in mean total symptom score (MTSS), mean number of wheals (MNW) and mean pruritus score (MPS) following 6 weeks of treatment with cetirizine (n=31) or rupatadine (n=33) (Adapted118).
The study also used a visual analogue scale (VAS) to assess sedation with participants asked to score themselves on a scale from 0 (alert) to 100 (very sleepy). After six weeks, cetirizine was shown to produce a significant increase in the patients self-reported sedation compared with baseline (p=0.0004). Rupatadine did not induce a significant change in sedation levels after 6 weeks compared with baseline (p=0.2179)118.
Desloratadine
Since 2001, randomised, double-blind, placebo-controlled studies of desloratadine in patients with CSU have shown that once-daily dosing (5 mg) offers significant symptom improvements versus placebo. Furthermore, the adverse event profile, including somnolence, was similar between the desloratadine and placebo groups119–121. Desloratadine has also been shown to improve the quality of life of patients with CSU. Using the DLQI, 77% of patients had a clinically significant improvement in the DLQI score after 42 days of treatment122. Interestingly, a third of patients in the study experienced complete symptom relief whereas 10% received no benefit from desloratadine treatment highlighting the heterogeneity in patient response to antihistamine treatment122.
Further studies have investigated whether desloratadine can be prescribed on an as-needed basis rather than continuously123,124. Patients taking daily desloratadine had significant improvements in quality of life compared with as needed dosing, supporting the recommendation for continuous antihistamine treatment in patients with CSU123,124.
Head-to-head
While the efficacy of desloratadine has been confirmed versus placebo, a number of head-to-head trials have sought to compare the efficacy of different second-generation H1-antihistamines.
A series of studies have compared desloratadine with levocetirizine in patients with CSU. In a 4-week, multicentre, randomised, double-blind study of 886 patients with CSU, levocetirizine was shown to significantly decrease pruritic severity to a greater degree than desloratadine125. Furthermore, levocetirizine significantly increased the patients’ global satisfaction over 1 and 4 weeks compared with desloratadine, while the safety and tolerability were similar between the treatments125. Similar results were observed in a separate study where 5 mg levocetirizine was observed to be more efficacious but appeared to have a greater sedative effect than 5 mg desloratadine126. However, the difference in sedative effect was only apparent in the first two weeks of treatment and so may be clinically manageable126.
Desloratadine was also compared to rupatadine in a prospective, randomised, single centre of 56 patients with CSU who were ≥12 years of age127. After four weeks of treatment, patients receiving rupatadine saw a significant improvement in total symptom score versus desloratadine (22.5% vs. 10.8%, P<0.001). Meanwhile, the Aerius Quality of Life Questionnaire (AEQLQ), a validated QoL measure for patients with skin diseases, established that both rupatadine and desloratadine treatment resulted in a significant improvement in QoL from baseline. However, the improvement observed with rupatadine was again significantly greater than that seen with desloratadine (31% vs. 17.7%, P=0.007). No difference was observed in the incidence of adverse events between the group127.
Ebastine
While a trial published in 2017 compared the effectiveness of ebastine and levocetirizine in patients with acute urticaria128, few studies have been undertaken in patients with CSU.
The earliest clinical trial assessed the efficacy of ebastine in managing CSU compared to terfenadine, the first non-sedating antihistamine that was removed from the market due to cardiovascular concerns, and placebo129,130. In the 3-month study, ebastine provided significantly greater symptom improvements than placebo and similar improvements to terfenadine. Symptoms were considered to have improved in 73% of ebastine patients, 68% of terfenadine patients and 52% of placebo patients129.
Fexofenadine
Fexofenadine is the active metabolite of terfenadine and has been used for the management of CSU for nearly 20 years. Two double-blind, placebo-controlled trials assessed multiple doses of fexofenadine in patients with CSU and moderate-to-severe pruritus over a 4-week period131,132. Over the course of each study, all doses of fexofenadine were shown to improve CSU symptoms and lessen their impact on sleep and daily activities compared with placebo. However, doses of 60 mg twice daily and above were significantly more effective than 20 mg twice daily and had a similar adverse event profile131,132. Similar efficacy was also observed in a 6-week study of 108 Thai patients with CSU given 60 mg fexofenadine twice daily133.
Twice-daily treatment with 60 mg fexofenadine was also shown to significantly improve DLQI scores versus placebo with fexofenadine treated patients demonstrating superior work productivity, performance of daily activities and a trend towards improved classroom productivity in the small number of school-aged patients (n=26)134.
While much of the early assessment of fexofenadine was done using 60 mg twice daily, the current approved dosage in patients with CSU is typically 180 mg once daily135. The use of 180 mg once daily was evaluated in in 255 patients with CSU over a 4-week period. This multicentre, randomised, double-blind, placebo-controlled study showed that once-daily fexofenadine could effectively manage CSU symptoms while remaining well tolerated136. A separate study in 254 CSU patients assessed the impact of 180 mg, once-daily fexofenadine on the DLQI and Work Productivity and Activity Impairment questionnaires over 4 weeks of treatment. Patients who received fexofenadine had significantly reduced work productivity impairment, overall work impairment and activity impairment compared with patients given placebo137.
Head-to-head
There are currently a lack of high-quality head-to-head trials assessing the comparative efficacy of fexofenadine in patients with CSU.
However, an earlier comparison of fexofenadine (180 mg once daily) with cetirizine (10 mg once daily) suggested that fexofenadine was inferior to cetirizine at managing CSU symptoms. In this study of 97 patients, 51.9% of subjects treated with cetirizine were symptom free at 4 weeks compared to 4.4% of fexofenadine patients. Furthermore, while 11.5% of cetirizine patients saw no improvement, this was as high as 53.3% of patients receiving fexofenadine138. While the study was randomised and double-blinded, uncertainty over the measures used to evaluate patients and the absence of baseline symptom severity scores suggest that the data in this study should be interpreted with caution139.
Levocetirizine
Levocetirizine is the levo-enantiomer of cetirizine. Oral 5 mg once-daily dosing of levocetirizine was shown to significantly improve CSU symptoms and patient quality of life over a 6-week treatment period versus placebo140. A comparable study that evaluated treatment over 4 weeks observed that levocetirizine was superior to placebo after 1 week and this was maintained over the course of the study141.
Head-to-head
With levocetirizine being the laevorotary enantiomer of cetirizine, it would be beneficial to know if there are any significant differences between the two therapies when managing CSU. A small study treated CSU patients sequentially with levocetirizine and cetirizine for 6 weeks. While the clinical efficacy was comparable, levocetirizine was reported to have a slightly better antipruritic effect, but with a probable increase in sedation compared with cetirizine142.
Levocetirizine has been compared to a number of other second-generation antihistamines:
Levocetirizine versus bilastine
A total of 525 adult patients with CSU were included in a multicentre, randomised, double-blind, placebo-controlled study to assess the efficacy and safety of 5 mg levocetirizine, 20 mg bilastine and placebo over 4 weeks. Bilastine and levocetirizine were both significantly more effective than placebo at improving the mean total symptom score from day two onwards (Figure 24).
Figure 24. Mean improvement in total symptom score as recorded every 12 hours over 28 days in patients with a documented history of chronic urticaria (Adapted114). SE, standard error.
Bilastine and levocetirizine were also similarly effective at reducing the individual symptoms of CSU although levocetirizine produced a significantly greater change from baseline in the maximum size of hives compared with bilastine.
The most commonly reported drug-related adverse events were headache and somnolence, but the overall and drug-related incidence of adverse events was not significantly different between the treatment groups114.
Another, smaller study published in 2020 also compared the safety, efficacy and tolerability of bilastine 20 mg and levocetirizine 5 mg in adult patients with moderate-to-severe CSU over a period of 42 days. In this double-blind, randomised controlled trial, a significant improvement in UAS7, DLQI, and VAS was reported in both groups at Day 42 compared to baseline. Overall, greater improvements were observed in the bilastine group, though this difference was only significant with respect to UAS7 reduction (p=0.03). No serious adverse effects were reported in either group, though sedation was significantly less among those treated with bilastine (p=0.04)143.
Levocetirizine versus desloratadine
A multicentre, randomised, double-blind study treated 886 patients with CSU with levocetirizine or desloratadine for 4 weeks. Levocetirizine treatment resulted in a significantly greater reduction in pruritus severity, pruritus duration and mean CSU symptom scores over the course of the study compared with desloratadine. While levocetirizine appeared to be more efficacious, the safety and tolerability of the treatment was comparable125.
Levocetirizine versus loratadine
To assess whether levocetirizine and loratadine offered different treatment outcomes in patients with CSU, a randomised, open-label, outdoor-based study was performed with 60 patients. Using the total symptom score (TSS) as the efficacy measure, levocetirizine was shown to produce a significantly greater decrease in TSS than loratadine (13.32% vs. 4.85%, p<0.001) after four weeks. The incidence of adverse events was comparable between the two treatment groups144.
Levocetirizine versus rupatadine
Rupatadine is a more recently developed H1-antihistamine that is approved for the management of allergic rhinitis and urticaria. To compare its efficacy and safety to levocetirizine in patients with CSU, a randomised, single-blinded, parallel-group, outdoor-based clinical study was performed in 54 patients. While both levocetirizine and rupatadine significantly reduced the total symptoms score (TSS), the observed effect was significantly greater with rupatadine than levocetirizine (Figure 25).
Figure 25. Mean change in TSS from baseline to Week 4 (Adapted145). TSS, Total Symptom Score.
The Aerius Quality of Life Questionnaire (AEQLQ) was also used to assess any improvements in the patients’ quality of life. Using a 25% reduction in AEQLQ as a cut-off for a clinically meaningful improvement found 69% (18/26) of rupatadine-treated patients achieved a meaningful improvement in quality of life versus 21% (6/28) of levocetirizine-treated patients. Meanwhile, the incidence of adverse events was found to be comparable145.
Loratadine
Loratadine has been available for many years and is on the World Health Organization’s list of essential medicines146. It is a widely used and available second-generation H1-antihistamine.
Head-to-head
Comparison of loratadine to older antihistamines have shown that it has comparable efficacy but without the sedative effects that first-generation antihistamines are associated with147,148.
Loratadine has also been compared to levocetirizine in the management of CSU. In a randomised, open-label, outdoor-based study of 60 patients, levocetirizine was shown to produce a significantly greater decrease in TSS than loratadine (13.32% vs. 4.85%, p<0.001) after four weeks. The incidence of adverse events was comparable between the two treatment groups144.
Rupatadine
Rupatadine is a second-generation H1-antihistamine that offers a novel combination of potent platelet-activating factor (PAF) and histamine antagonism149,150.
Following approval in the management of allergic rhinitis, the first publication evaluating treatment potential of rupatadine in patients with urticaria was published in 2007. In a 4-week phase II, randomised, double-blind, placebo-controlled, multicentre study of 277 patients with CSU, rupatadine was observed to offer a dose-dependent reduction in urticaria symptoms.
A subsequent randomised, double-blind, placebo-controlled, parallel-group, multicentre study assessed the efficacy of 10 mg and 20 mg rupatadine over 4 weeks in 333 patients with moderate-to-severe CSU. The primary outcome was the change in mean pruritus score from baseline and this was observed to be 44.5% for placebo and 57.5% (p<0.005) and 63.3% (p<0.0001) for 10 mg and 20 mg rupatadine respectively. A fast onset of action was also observed with significant reductions in CSU symptoms observed from the first dose i.e., within 24 hours. Treatment with rupatadine also significantly improved patients’ quality of life as measured by the DLQI and the mean total symptom score (Figure 26)151.
Figure 26. Percentage reduction from baseline in mean total symptom score over 4- and 6-weeks following treatment with placebo, rupatadine 10 mg or rupatadine 20 mg (Adapted151). MTSS, mean total symptom score; Rup, rupatadine.
The most frequently reported adverse events were headache (8.0%, 4.5%, 8.3% for placebo, 10 mg and 20 mg rupatadine) and somnolence (5.3%, 2.7%, 8.3% for placebo, 10 mg and 20 mg rupatadine). The outcomes in this study indicated that 10 mg rupatadine offered similar efficacy outcomes to a 20 mg dose, but with better tolerability, in particular an incidence of somnolence comparable to placebo151. Further assessments have indicated rupatadine is a well-tolerated drug with no CNS or cardiovascular effects152.
More recently, a double-blind, randomised, multicenter, placebo-controlled clinical trial published in 2019 investigated the safety and efficacy of rupatadine in adult and adolescent patients with CSU153. Patients orally received either rupatadine 10 mg (n=91), rupatadine 20 mg (n=92) or placebo (n=94) once a day for 14 days153. The least squares mean total pruritus score (TPS) difference was -1.956 and -2.121 for rupatadine 10 mg versus placebo and rupatadine 20 mg versus placebo respectively (analysis of covariance, p<0.001 for both)153. Overall, the results of this study supported the use of rupatadine at 10 mg or 20 mg over placebo153. While no clinically significant adverse events were observed, it is worth noting that a dose-related increase in the incidence of adverse drug reactions was reported153.
Data also supports the efficacy of rupatadine within the real-world clinical setting. An open, prospective, non-interventional study across 146 German clinics identified 660 patients with CSU with the majority receiving 10 mg rupatadine once (n=477) or twice (n=105) daily154. At the end of their treatment, 93.2% (606/650) of patients reported an improvement in overall symptoms while a reduction in the frequency and severity of angioedema episodes was also observed. Furthermore, all domains of the CU-Q2oL quality of life questionnaire were improved while 95.7% of patients, and 87.8% of physicians, rated tolerability as very good or good154.
Efficacy of first-line rupatadine in CSU
The efficacy and safety of rupatadine has also been compared to the levo-enantiomer of cetirizine, levocetirizine. In a randomised, single-blinded, parallel-group, outdoor-based clinical study of 54 patients with CSU, both levocetirizine and rupatadine significantly reduced the TSS, although the observed effect was significantly greater with rupatadine than levocetirizine (Figure 27).
Figure 27. Mean change in TSS from baseline to Week 4 (Adapted145). TSS, Total Symptom Score.
Using a 25% reduction in the AEQLQ as a cut-off for a clinically meaningful improvement found 69% (18/26) of rupatadine-treated patients achieving a meaningful improvement in quality of life versus 21% (6/28) of levocetirizine-treated patients. Meanwhile, the incidence of adverse events was found to be comparable145.
Comparing the efficacy of second-generation H1-antihistamines
As discussed earlier in this section, high-quality clinical data on the comparative efficacy of different second-generation H1-antihistamines is scarce. This was highlighted in recent a network meta-analysis carried out by Phinyo and colleagues. This study aimed to investigate the comparative efficacy of second-generation H1-antihistamines at their licensed doses in patients with CSU112.
Three groups of treatment options were identified using a cluster ranking based on Surface Under the Cumulative Ranking (SUCRA) values for changes in total symptom score from baseline and acceptability outcome via any cause of dropout. The first included olopatadine, fexofenadine and rupatadine, all of which were found to have high efficacy and moderate acceptability. The second group included mizolastine, bilastine, levocetirizine, loratadine and desloratadine, which had high acceptability and moderate efficacy. The third group included cetirizine, which had the lowest SUCRA ranking, as well as placebo (Figure 28)112.
Figure 28. Cluster ranking based on Surface Under the Cumulative Ranking (SUCRA) values for changes in total symptom score from baseline and acceptability outcome via any cause of dropout (Adapted112). BILA, bilastine; CETI, cetirizine; DESL, desloratadine, FEXO, fexofenadine; LEVO, levocetirizine; LORA, loratadine; MIZO, mizolastine; OLOP, olopatadine; PLAC, placebo; RUPA, rupatadine.
The study concluded that while olopatadine, fexofenadine, bilastine, rupatadine and levocetirizine all demonstrated superior therapeutic efficacy compared to placebo, the quality of almost all studies included was low to very low, and the authors called for more rigorous head-to-head trials in the future to confirm these findings112.
The unmet medical need in CSU
H1-antihistamines are ineffective in many patients with CSU145
- Second generation H1-antihistamines fail to control symptoms in up to 50% of CSU patients at licensed doses145
- Guidelines recommend an up to four-fold dose increase of H1-antihistamine in patients with an insufficient response at licensed doses18
- It has been suggested that the increase in dose not only blocks histamine mediated effects, but also reduces mast cell activation and has an impact on various cytokine and endothelial adhesion molecules155
- Up-dosing of H1-antihistamines improves treatment responses, but up to one third of patients remain symptomatic5,18
Some second generation H1-antihistamines (e.g. cetirizine, loratadine) potentially cause sedation when licensed doses are exceeded156–160.
Second-line therapy for CSU
The EAACI/GA2LEN/EDF/WAO urticaria guidelines recommend increasing the dose of second generation H1-antihistamines up to four-fold if symptoms of urticaria persist at licensed doses18
Should we up-dose second-generation H1-antihistamines?
According to the EAACI/GA2LEN/EDF/WAO guidelines, the recommended second-line therapy for patients who remain symptomatic despite first-line treatment is an increase in the dose of a second-generation H1-antihistamine up to four-fold the licensed dose18. A recent retrospective analysis of 178 patients lends some support to this approach with 30% of those requiring up-dosing achieving a sufficient response161. Meanwhile, a retrospective patient survey (N=319) offers further support with 40%, 42% and 54% reporting significant treatment benefits with two-, three-, and four-fold up-doing respectively. Importantly, the levels of reported adverse events and sedation were not significantly different from those observed with standard doses162. However, data on antihistamine up-dosing remains insufficient for many second-generation H1-antihistamines.
A 2015 comparative analysis included 12 published studies to evaluate the effectiveness of up-dosing different antihistamine in urticaria patients. The analysis concluded that there was no significant difference in efficacy between fexofenadine and bilastine, rupatadine and bilastine, and desloratadine and levocetirizine. However, fexofenadine, rupatadine and bilastine had significantly higher efficacy than desloratadine and levocetirizine, and rupatadine had higher efficacy than fexofenadine163.
40–50% of patients with CSU reported significant benefit from taking two- to four-fold doses of second-generation H1-antihistamines162
Within the guidelines, bilastine, cetirizine, desloratadine, levocetirizine, fexofenadine and rupatadine are cited as having studies verifying their use at increased doses18.
Clinical evidence
While the guidelines currently recommend up-dosing of second-generation H1-antihistamines for patients who remain refractory to licensed doses, some of the evidence comes from studies in patients with chronic inducible urticaria18. Here we provide an overview of the available data in patients with CSU.
Cetirizine
The evidence for up-dosing cetirizine for CSU remains quite weak164. In a small study of 22 patients with moderate-to-severe chronic urticaria who were unresponsive to licensed doses of cetirizine, up-dosing of cetirizine to 30 mg a day for one week resulted in only one patient responding satisfactorily165. However, the short timeframe of this study may limit the outcomes. CSU symptoms were significantly improved in patients (N=21) who were up-dosed and maintained on 20 mg daily compared with patients who were up-dosed for 1–2 weeks and then stepped back down to 10 mg daily166.
Desloratadine and levocetirizine
A double-blind, randomised, two parallel-armed study investigated the efficacy of weekly up-dosing of desloratadine or levocetirizine in 80 patients with CSU. Patients who became symptom-free left the study while those who continued to experience symptoms were up-dosed until reaching four-fold dosing (20 mg desloratadine or 20 mg levocetirizine). Patients who remained symptomatic after one week of four-fold treatment were switched to 20 mg of the alternative medication. Increasing the prescribed dose above 5 mg more than doubled the success rate for both treatments. However, significantly more patients were successful with levocetirizine than desloratadine and a number of patients who remained symptomatic with desloratadine achieved disease control after switching to levocetirizine (Figure 29). Patients receiving levocetirizine also reported a significantly greater improvement in discomfort from urticaria than patients given desloratadine167.
Figure 29. Percentage of patients whose symptoms were relieved by desloratadine (N=40) or levocetirizine (N=40) over the four weeks of the study. 17.5% (n/N=7/25) of patients switched from 20 mg desloratadine to 20 mg levocetirizine became symptom free. Zero patients switched from 20 mg levocetirizine to 20 mg desloratadine (n=18) became symptom free (Adapted167).
Somnolence is a significant concern when up-dosing antihistamines. Interestingly, patients reported reduced somnolence as the study went on and the antihistamine dose increased. The authors suggested that reduced urticaria symptoms and discomfort resulted in better sleep and a subsequent improvement in daytime wakefulness. Another possibility they highlight is the development of CNS tolerability to the sedative effects of both drugs.
Finally, the incidence of adverse events in this study was low and were unlikely to be associated with either treatment. No adverse events were serious enough to cause discontinuation and no changes in ECG were observed167.
A 2015 comparative study included the Staevska et al. publication within its analysis163,167. Comparison of desloratadine, levocetirizine and bilastine up-dosing revealed that significantly more patients were symptom free following treatment with bilastine (Figure 30)163.
Figure 30. Percentage of patients who were symptom free (Adapted163). QD, once daily.
Fexofenadine
While the approved dose for fexofenadine in patients with CSU is 180 mg once daily, two double-blind, placebo-controlled trials evaluated the efficacy of fexofenadine at doses of 20, 60, 120 and 240 mg twice daily131,132.
Over a 4-week trial period in 439 patients with moderate-to-severe CSU, fexofenadine produced a significant improvement in the primary efficacy outcome, mean pruritus score, compared with placebo. However, the dose response was not established beyond treatment with 60 mg twice daily (Figure 31).
Figure 31. Mean change in mean pruritus score from baseline over 4 weeks with different doses of fexofenadine (Adapted131). BID, twice daily.
While increasing the dose of fexofenadine beyond 60 mg twice daily offered small improvements in symptom relief for patients, it is worth noting that the reported adverse events were consistent across the different treatment doses and continued to be comparable to placebo131. Similar treatment outcomes and adverse event profiles were observed in another clinical trial involving 418 patients with CSU indicating that higher doses of fexofenadine offer a comparable safety profile to licensed doses but with only a modest increase in efficacy132.
The comparative analysis of trials examining up-dosing of second-generation H1-antihistamines by Sànchez-Borges et al. included the Finn et al. and Nelson et al. studies131,132,163. Comparing the percentage of patients who achieved a mean pruritus score outcome indicated that fexofenadine up-dosing to 120 mg or 240 mg twice daily was significantly less effective than rupatadine up-dosing to 20 mg once daily (p<0.0001 and p=0.03, respectively)163.
Rupatadine
While the licensed dose of rupatadine for patients with CSU is 10 mg once daily, clinical trials in this patient population have included doses up to 20 mg. In both of these studies, a trend towards improved outcomes was observed with increased treatment doses, but they did not reach statistical significance between doses151,168.
Up-dosing of rupatadine
A responder analysis pooled the results from the two trials to assess whether 20 mg of rupatadine offered clinically meaningful benefits over 10 mg rupatadine. In this study, significantly more patients achieved a ≥75% reduction from baseline in mean pruritus score, mean number of hives and mean UAS over the 4-week study periods (Figure 32169).
Figure 32. Percentage of patients achieving a ≥50% and ≥75% improvement in mean pruritus score after four weeks of treatment (Adapted169). PBO, placebo; RUP, rupatadine.
While the responder analysis suggests that 20 mg rupatadine once daily offers improved treatment outcomes compared with 10 mg once daily, the initial clinical studies suggest that the safety profile of the lower dose may be preferable151,168,169. Both clinical studies observed a modest increase in somnolence in the 20 mg treatment group151,168. However, within a real-world clinical setting this may be manageable. In a prospective, non-interventional trial involving 660 patients across 146 German dermatology clinics, 17.2% received the higher 20 mg daily dose of rupatadine. Of these patients, 76% maintained this dose and 7.3% increased it further, suggesting that higher doses of rupatadine are tolerable for the majority of patients154.
The 2015 comparative analysis by Sànchez-Borges et al. also included the rupatadine studies151,163,168. In the analysis, up-dosing of rupatadine was found to significantly improve the mean pruritus score versus fexofenadine 120 mg and 240 mg (Figure 33)163.
Figure 33. Comparison of fexofenadine and rupatadine up-dosing as assessed by patients achieving outcomes in mean pruritus score (Adapted163).
Third- and fourth-line therapy for CSU
The EAACI/GA2LEN/EDF/WAO urticaria guidelines recommend omalizumab as add-on therapy to second-line treatment if symptoms of urticaria persist. For exacerbations a short course (maximum 10 days) of glucocorticosteroids can be considered18.
Omalizumab is an anti-immunoglobulin E (IgE) humanised monoclonal antibody160. Omalizumab 300 mg is approved in the European Union and the United States of America for the treatment of CSU (also known as chronic idiopathic urticaria [CIU]) in adult and adolescent (12 years and above) patients with inadequate response to H1-antihistamine treatment or who remain symptomatic despite H1-antihistamine treatment170,171.
Clinical evidence
Aside from the standard treatment using second-generation H1-antihistamines, a number of alternatives are available for cases where the patient is refractory or intolerant to these medications. Here we present the evidence from clinical studies on the available treatment options, including omalizumab, ciclosporin, montelukast, oral corticosteroids and other less proven medications.
Omalizumab
Results from the three pivotal Phase III trials, ASTERIA I, ASTERIA II and GLACIAL supported the efficacy, safety and tolerability of omalizumab in refractory CSU172–174.
Efficacy
Omalizumab significantly improved itch versus placebo in Phase III studies of refractory CSU patients (Figure 34)172–174.
Figure 34. Improvement in itch severity score (ISS) was significantly greater with all doses of omalizumab vs. placebo at Week 12 (mITT) (Adapted172–174). ISS, Itch Severity Score ; mITT, modified intention-to-treat.
In these Phase III studies, omalizumab 300 mg consistently provided significant improvements in the symptoms of CSU compared with placebo172–174.
- A significant proportion of patients became either completely free of itch and hives (range 34–44%; p<0.001 to p<0.0001) or had their symptoms suppressed to levels described as ‘well controlled disease’ at 12 weeks (52–66%, p<0.0001)172–175.
- Weekly itch severity score (ISS) was significantly reduced by 62–71% from baseline to Week 1, with itch relief being rapid and sustained throughout the treatment period172–174,176.
- The Dermatology Life Quality Index (DLQI) was significantly reduced (i.e. QoL improved) at 12 weeks in ASTERIA I (-10.3 vs. -6.1, p=0.0001), ASTERIA II (-10.2 vs. -6.1, p=0.0004) and GLACIAL (-9.7 vs. -5.1, p<0.001), corresponding to a 74%, 78% and 73% reduction vs. baseline172–175.
- The weekly hives score was significantly reduced (i.e. fewer hives) at 12 weeks in ASTERIA I (-11.4 vs. -4.4, p<0.0001), ASTERIA II (-12.0 vs. -5.2, p<0.001) and GLACIAL (-10.5 vs. -4.5, p<0.001), corresponding to a 67%, 74% and 62% reduction vs. baseline172–175.
- Proportion of angioedema-free days over weeks 4–12 were significantly increased in GLACIAL (91% vs. 88.1%, p<0.001)174.
Results from the open-label Phase IV SUNRISE study of omalizumab in adults with CSU (n=136) nonresponsive to H1-antihistamine therapy suggest that approximately 75% of patients achieve disease control after 12 weeks of treatment177.
The Phase IV Xolair Treatment Efficacy of Longer Duration in Chronic Idiopathic Urticaria (XTEND-CIU) study was a multicentre, randomised, double-blind, placebo-controlled trial including CSU patients (n=205) between the ages of 12 and 75 years. After a 24-week open-label period of omalizumab 300 mg every four weeks, patients were stratified by 7-day Urticaria Activity Score (UAS7): a UAS7 of ≤6 (protocol-defined responder) vs UAS7 >6 (non-responders). Responders subsequently entered a 48-week double-blind phase and were assessed for clinical worsening vs placebo (Figure 35)178.
Figure 35. XTEND-CIU study design (Adapted178). CIU, chronic idiopathic urticaria; CSU, chronic spontaneous urticaria; UAS7, 7-day Urticaria Activity Score; Q4W, every four weeks; XTEND, Xolair Treatment Efficacy of Longer Duration in Chronic Idiopathic Urticaria.
During the open-label phase, patients with moderate-to-severe CIU symptoms at baseline demonstrated improvements in the UAS7 as early as Week 1, which continued to Week 24. In total, 73.0% and 52.0% of patients were considered as responders (UAS7 ≤6) or complete responders (UAS7 = 0) at Week 24, respectively.178.
Chronic spontaneous urticaria was formerly known as chronic idiopathic urticaria and the two terms are used interchangeably in this section42
During the double-blind period, most placebo-treated patients (60.4%) experienced CSU relapse vs 21% with omalizumab, with differences between groups reported as early as 1 month and continuing to increase until the end of the treatment period (p<0.0001) (Figure 36)41.
Patients who continued on omalizumab beyond 24 weeks maintained symptom control (demonstrated by clinically insignificant change in mean UAS7). These patients also experienced a lower number of angioedema days vs placebo (average percentage of angioedema days, 0.8% vs 7.3%, respectively). There were no new safety signals during the double-blind period, and no evidence of allergic reactions associated with stopping and restarting omalizumab41.
Figure 36. Percentages of patients experiencing CSU clinical worsening (defined by UAS7 ≥12 for two consecutive weeks) and Dermatology Life Quality Index (DLQI) worsening (≥3-point increase in the DLQI score) were significantly lower in with omalizumab vs placebo (Adapted41).
The XTEND-CIU study demonstrated that continued treatment with omalizumab was beneficial to patients by preventing return of symptoms and achieving sustained control41.
A recent systematic literature review for the EAACI Biologicals guidelines highlighted the efficacy and safety of omalizumab. Ten randomised controlled trials were included, comprising 1,620 subjects (aged 12-75 years) treated with omalizumab for 16 to 40 weeks. Results demonstrated the clinically meaningful improvements in urticaria activity (UAS7 scoring), itch severity (ISS7 scoring), and QoL associated with omalizumab 300 mg179.
It has been suggested that response to omalizumab may be tracked in patients by measuring levels of basophil FcƐRI180. Treatment with omalizumab leads to significant reductions in FcƐRI receptor density on basophils, which may be detected within one week of therapy and persist for two months following treatment cessation; indicating a cellular treatment effect181.
Measuring the baseline levels of these high-affinity IgE receptors may also play a role in determining potential response180. Interestingly, further research into predictors of response to omalizumab and relapse in patients with CSU found that total IgE, but not total D-dimer, correlated with treatment response182. Total IgE levels were significantly higher in responders compared with non-responders (p=0.002), but neither biomarker correlated with either first or second relapse182.
Omalizumab resistance is not well-understood. However, a number of factors associated with resistance have been identified in patients with severe CSU. These include183:
- obesity
- arterial hypertension
- high plasma C3 levels
- high C-reactive protein (CRP) levels
Safety
In Phase III studies, omalizumab 300 mg was well tolerated in over 700 patients with refractory CSU172–174, with no safety issues or concerns compared with the known safety profile of omalizumab for the treatment of moderate-to-severe allergic asthma184.
- Safety data were similar for omalizumab-treated vs. placebo-treated patients, with no major differences in incidence of adverse events or serious adverse events172–174.
- In pooled data for all three studies from Weeks 0–12, the only drug-related adverse reactions occurring more frequently with omalizumab 300 mg vs. placebo were sinusitis (4.9% vs. 2.1%), headache (6.1% vs. 2.9%) and arthralgia (2.9% vs. 0.4%)184.
In pooled data from ASTERIA I and GLACIAL from Weeks 0–24, the only drug-related adverse reaction occurring more frequently with omalizumab 300 mg vs. placebo was upper respiratory tract infection (5.7% vs. 3.1%)184.
A recent systematic literature review for the EAACI Biologicals guidelines demonstrated that omalizumab 300 mg was associated with decreases in rescue medication use and drug-related serious adverse events179.
Fourth-line therapy
If adequate control is not achieved within six months, or earlier if symptoms are intolerable, ciclosporin can also be used as an alternative add-on therapy to second-generation H1-antihistamines18.
Ciclosporin A
The efficacy of ciclosporin A in the treatment of urticaria has been well documented over 20 years in randomised, double-blind, placebo-controlled trials as well as open controlled trials108,176. Its efficacy has also been shown in combination with second generation H1-antihistamines184. However, it is not licensed in this indication, is associated with a high frequency of adverse events, and is only recommended for patients with severe disease who are refractory to any dose of antihistamine175. Currently, there are no reliable, practical biomarkers for predicting treatment response to ciclosporin treatment185.
A 2015 study in the UK gathered real-world evidence of third-line treatment options for CSU to facilitate clinical decision-making186. Treatment and safety outcomes of CSU patients treated with either omalizumab or ciclosporin in UK secondary care were investigated. Based on clinician comments and DLQI scores, symptoms and quality of life showed a greater improvement in the omalizumab-treated cohort than in the ciclosporin-treated cohort. It was also concluded that validated patient-reported measures of disease severity and quality of life should be used routinely in CSU management.
Oral corticosteroids
The efficacy of oral corticosteroids for the treatment of CSU has been well documented184,187. There are a number of severe adverse effects associated with long-term systemic use of corticosteroids, including osteoporosis, hypertension and diabetes mellitus184.
For acute urticaria and acute exacerbations of CSU a short course of oral corticosteroids (up to 10 days) is therefore recommended.
Other treatments
There are other treatments that are not recommended by the international guidelines but may have relevance for some healthcare systems with fewer financial resources108.
Table 5. Clinical evidence for other treatment options (Adapted108). CSU, chronic spontaneous urticaria; UV-A, ultraviolet A; PUVA, psoralen with UV-A; UV-B, ultraviolet B; nb-UVB, narrowband UV-B.
| Treatment | Notes |
| H2-agonists | Evidence is too low to recommend but they may be relevant in some less-developed countries |
| Dapsone | Evidence is too low to recommend but they may be relevant in some less-developed countries |
| Sulfasalazine | Only trials of low quality or case series have been published |
| Methotrexate | Only trials of low quality or case series have been published |
| Interferon | Only trials of low quality or case series have been published |
| Plasmapheresis | Only trials of low quality or case series have been published |
| Phototherapy | Only trials of low quality or case series have been published |
| Intravenous immunoglobulins | Have been successfully used in case reports. Recommended only for use in specialised centres as last option |
| Tumour necrosis factor a antagonists | Have been successfully used in case reports. Recommended only for use in specialised centres as last option |
| Phototherapy | For CSU: UV-A, PUVA and UV-B (nb-UVB) treatment for 1–3 months can be added to antihistamine treatment |
| Tranexamic acid | Treatments shown to be ineffective in CSU |
| Sodium cromoglicate | Treatments shown to be ineffective in CSU |
| Montelukast | The efficacy of montelukast has been the most successful of randomised control trials investigating leukotriene receptor antagonists. However, the evidence is too low for this treatment to be recommended as an add-on to H1-antihistamines |
Real-world data
Clinical trial data offer a critical appraisal of a treatment’s efficacy and safety. However, exclusion criteria commonly result in a patient population that does not necessarily reflect society and the patients that are typically seen in the clinic. Real-world data can provide vital insights into the performance of a drug in the clinical setting.
First- and second-line treatment options
Bilastine
While some patients achieve disease control with licensed doses of second generation H1-antihistmaines, many require up-dosing to up to four-fold licensed doses. A small open-label study of 29 patients with CSU who did not respond sufficiently to first-line treatment assessed the efficacy of up-dosing bilastine. Patients were treated with 20 mg bilastine orally each day and after two weeks. If the response was insufficient, their dose was doubled to 40 mg and finally 80 mg. A significant reduction in mean Weekly Urticaria Activity Score (UAS7) was observed between baseline and 20 mg bilastine and between 20 mg and 40 mg bilastine. However, no further significant reduction was observed following up-dosing to 80 mg bilastine (Figure 37) with 11 patients continuing to have uncontrolled disease activity despite receiving the highest possible dose of anti-histamine188.
Figure 37. Mean UAS7 levels following treatment with bilastine and subsequent up-dosing in patients with an insufficient response to the lower dose (Adapted188). UAS7, 7-day Urticaria Activity Score.
Desloratadine
A post-marketing surveillance study assessed the efficacy and safety of desloratadine in 9,246 patients with CSU. A significant improvement in symptoms was observed from baseline (p<0.0001) and 67% and 71% of patients reported improvements in sleep and daily activities, respectively (p<0.0001). For patients that had received prior antihistamine treatment, over half rated the onset of efficacy of desloratadine to be faster than cetirizine (55.5% of patients), loratadine (54.7%) and fexofenadine (57.6%). Importantly, only 0.5% of patients experienced adverse events and no serious adverse events were reported189.
Further insight into the real-world efficacy and safety of desloratadine was provided by an overview of four post-marketing surveillance studies (including the Augustin & Ehrle study) that reported on 77,880 patients aged ≥12 years who were being treated for CSU or seasonal allergic rhinitis. In these four studies, the number of patients reporting no itching increased from 53.2% at baseline to 80.3% following desloratadine treatment. Furthermore, those reporting moderate or severe itch decreased from 30.1% to 3.7% after treatment. Across all four studies, just 0.37% of patients reported an adverse event with the most common being fatigue, headache, dry mouth and nausea190.
Levocetirizine
With ethnic/genetic differences being shown to influence the pharmacokinetics of loratadine191, an open-label, observational study sought to investigate the efficacy of levocetirizine for patients with allergic rhinitis or urticaria in Taiwan (236 patients with AR and 97 patients with urticaria). Treatment with 5 mg levocetirizine once daily was shown to be efficacious with 60–80% of patients with CSU reporting complete or marked improvements in symptoms. Levocetirizine was also generally well-tolerated with 9.6% of patients reporting a treatment-emergent adverse event with somnolence (7.2%), fatigue (4.5%) and dry mouth (1.8%) most commonly reported192.
Rupatadine
The real-world efficacy and safety of rupatadine in patients with CSU was assessed in a prospective, non-interventional trial of 660 patients who presented to the clinic of one of 146 dermatologists in Germany. Treatment with 10 mg rupatadine once- or twice-daily was assessed for a median treatment period of 28 days. An overall improvement in symptoms, as assessed by UAS7 scores, was seen in 93.2% of patients with the median UAS7 being reduced from 25.0 at baseline to 7.0 after treatment. Importantly, both dermatologists and patients reported similar ratings on the global efficacy of rupatadine (Figure 38). Rupatadine also improved angioedema outcomes with 60.9% of patients affected by angioedema showing a complete response and 31.6% reporting a clinically meaningful improvement in occurrence and severity of episodes154.
Figure 38. Physician and patient global assessment of treatment effectiveness (Adapted154).
Meanwhile, 95.7% of patients and 87.8% of physicians reported the tolerability of rupatadine to be very good or good. In this study, 3.2% of patients reported an adverse event with fatigue, headache and nausea being the most frequently reported events that may be associated with treatment154.
Third-line treatment options
Omalizumab received approval for the treatment of CSU in the USA and Europe in 2014193,194
A United States-based study utilised insurance claims data between the start of 2013 and July 2016 to assess the real-world use of omalizumab in patients with CSU195. Of the 1,546 patients included in the analysis, 84.5% initiated treatment with a 300 mg dose, although this increased with time (80.0% in 2014 versus 92.3% in 2015). A large majority (90%) of patients who received a 300 mg dose of omalizumab were maintained on that dose while the mean duration of treatment was 273 days (± 113 days), the mean number of omalizumab administrations was 8.3 injections (± 4.8 administrations), and the mean frequency of administrations was 44 days (± 29 days). Nearly 50% of patients (47.4%) were treated continuously for 12 months and for those that discontinued for ≥3 months, 21% went on to restart treatment after an average of 4.4 months (± 1.3 months).Importantly, the proportion of patients taking additional medications for their CSU decreased with the largest decrease seen in the number of patients taking oral corticosteroids (75.7% at baseline vs. 49.9% after omalizumab initiation)195.
A similar reduction in the use of oral corticosteroids was seen in another US-based retrospective, observational cohort study investigating the real-world use of omalizumab in patients with CSU. In this smaller cohort of 88 patients, 83.7% of patients reported symptom improvement following omalizumab initiation and over 75% remained on treatment for 12 months or longer196. Meanwhile, in a third retrospective cohort study from the United States, approximately 60% of patients who received omalizumab were maintained on treatment for 18 months or longer, while 28.6% of those who discontinued restarted omalizumab treatment. As with the other real-world studies, the proportion of patients taking additional medications to control their CSU significantly reduced over time (Figure 39)197.
Figure 39. Concomitant medication use for patients with CSU before and after initiation of omalizumab treatment (N=298), use of all treatment categories were significantly reduced post omalizumab initiation (p<0.05) (Adapted197).
Some smaller studies have also sought to address the real-world efficacy of omalizumab in patients with CSU. In the UK, a retrospective observational study looked at the outcomes of 46 patients who received omalizumab. For patients with UAS7 scores available, a significant mean 25.4-point improvement was seen during treatment (p<0.0001) and 68% of patients achieved a UAS7 score of 0 signifying that they were symptom free. In addition, the impact on patient quality of life (QoL) was assessed. A mean Dermatology Life Quality Index (DLQI) score of 19.5 was observed at baseline, which reduced by a mean of 16.4 points (p<0.0001) after omalizumab treatment. Of these patients, 65% achieved a DLQI score of 0–1 indicating that the disease had no impact on their QoL. Potential adverse events were also reported with 37% of patients experiencing at least one event, most commonly skin reactions, angioedema and anaphylaxis. However, both cases of anaphylaxis occurred in the same patient who had a prior history of recurrent anaphylaxis and the episodes were reported as most likely being an exacerbation of the underlying CSU186. Meanwhile, a Greek retrospective analysis of 20 patients with refractory CSU looked at outcomes following omalizumab initiation. In this small cohort of patients, 85% observed a complete response while the remaining patients were considered well-controlled198.
A 2019 meta-analysis of real-world data, including 67 published reports on real-world effectiveness, suggest omalizumab therapy was associated with an average 25.6-point improvement in UAS7 scores, compared with a 14.9-22.1-point improvement reported in clinical trials. In addition, complete and partial response rates were reported at 72.2% and 17.8%, respectively, with an average adverse event rate of 4.0% (vs 2.9-8.0% reported in clinical trials). These results demonstrated that the benefits and safety of omalizumab in the real-world treatment of CSU met or exceeded the results obtained from clinical trials199.
The recommended fourth-line treatment for patients who fail to respond to omalizumab is ciclosporin as an add-on to second-line therapies. However, a number of real-world evidence studies have now been conducted with the aim of establishing the potential benefits of omalizumab up-dosing. These were analysed in a recent review article, which suggested that treatment with higher than standard doses of omalizumab may, in some cases, result in improvements in UAS7, UCT, and quality of life scores200.
Fourth-line treatment options
A UK-based retrospective analysis assessed the efficacy and safety of ciclosporin in 72 patients with CSU. Physician-rated responses indicated that 17% of ciclosporin-treated patients were symptom-free during treatment versus 55% who had improved symptoms and 28% who had no response. Improvement in QoL was also assessed with the mean DLQI score reduced by 8.9 points from baseline following treatment and 21% of patients achieving a DLQI score of 0–1. Adverse events were reported by 39% of patients with the most common being hypertension, fatigue/tiredness, gastrointestinal problems and headache186.
Patient journeys in CSU
Treatment escalation occurs in less than one-third of patients on antihistamines who would benefit from omalizumab therapy201
According to the 2017 EAACI/GA2LEN/EDF/WAO urticaria guidelines, first-line therapy for patients with CSU is standard-dosed second-generation, non-sedating H1-antihistamines18. If patients do not achieve adequate control after 2–4 weeks, or symptoms remain intolerable, physicians are advised to increase H1-antihistamine dose by up to four-times18. Unfortunately, a large proportion of patients remain symptomatic despite treatment with these drugs, with up to 60% failing to achieve adequate control of their disease at approved doses40. The recommended third-line treatment for patients who remain symptomatic following 2–4 weeks of treatment with up-dosed H1-antihistamines is omalizumab as an add-on therapy18.
One year data from the German arm of the AWARE study revealed that a large proportion of patients experienced long delays in receiving a diagnosis and their disease had, in many cases, remained uncontrolled for many years34. Escalation to second- and third-line treatments was much lower than anticipated, a finding that was attributed to perceived inconsistencies in the evidence of the benefits of up-dosing antihistamines, financial considerations, and lack of physician experience with omalizumab34.
In 2020, two-year data from the AWARE study showed that the proportion of patients being treated with either first- second- or third-line therapy remained constant (~77%)201. While use of standard-dosed and up-dosed H1-antihistamines decreased over the 24-month study period, omalizumab use increased, making it the most common treatment option used in this group of antihistamine refractory patients (Figure 40)201.
Figure 40. Therapy changes over the 2-year study period in the AWARE study201. H1-AH, H1-antihistamines.
Nevertheless, researchers noted that despite the increase in use of omalizumab, less than one in three patients who might have derived benefit from switching to third-line treatment actually did so201.
Treatment summary
The aim of therapy for CSU is rapid and complete symptom control18
The international EAACI/GA2LEN/EDF/WAO guidelines recommend the following step-wise approach to the treatment of urticaria18:
- Second generation H1-antihistamines at licensed doses.
- Second generation H1-antihistamines at up to four-fold increased dose, if symptoms persist.
- Only seven of the second generation H1-antihistamines have been proven with increased dosing (bilastine, cetirizine/levocetirizine, fexofenadine, loratadine/desloratadine and rupatadine)
- Addition of omalizumab in patients with an inadequate response to H1-antihistamines.
- If inadequate control in response to the addition of omalizumab within 6 months ciclosporin can be administered as an add-on to second generation H1-antihistamines. For severe exacerbations a short course (maximum 10 days) of glucocorticosteroids can be considered.
It is recommended to reassess the need for continued or alternative drug treatment every 3–6 months, as the severity and symptoms of urticaria may fluctuate and spontaneous remission may occur at any time108.
CSU Guidelines
Best practice guidelines for CSU have been developed and published by a number of national and international groups, and those with major significance are described in this section. Broadly speaking they recommend second generation antihistamines of standard and then increased dose, followed by alternative agents such as anti-inflammatories, immunosuppressants or biologics.
CSU presents as wheals and/or angioedema which usually last for less than 24 hours and resolve without leaving a mark. These symptoms occur for six weeks or more18.
The diagnosis of CSU is usually made clinically. Not all possible causative factors need to be investigated in all patients, however, a thorough history should be taken as the first step. The following should be reviewed18:
- onset of disease
- frequency of attacks
- provoking factors
- diurnal variation
- shape, size and distribution of wheals
- angioedema
- pain, itch, burning, fever
- family history
- allergies
- psychosomatic/psychiatric history
- surgical implantations
- gastrointestinal symptoms
- induction by physical stimulation
- drugs
- correlation to food
- correlation to menstrual cycle
- smoking
- work
- hobbies
- quality of life
- previous therapy (response)
- previous diagnostic results
Following the patient history, a physical examination of the patient should be made. This should include a diagnostic provocation test including drug, food and physical tests where indicated by the patient’s history. All subsequent diagnostic steps will depend on patient history and the nature of the urticaria subtype18.
EAACI Guidelines recommend only very limited routine diagnostic measures in chronic spontaneous urticaria18.
Routine
- C-reactive protein (CRP)/erythrocyte sedimentation rate (ESR)
- Differential blood cell count
Extended
- Infectious diseases
- Type I allergy
- Functional autoantibodies
- Thyroid hormones
- Physical tests
- Pseudo allergen free diet
- Autologous serum skin test (ASST)
Table 6. Recommended diagnostic tests in frequent spontaneous urticaria subtypes (Adapted18). CIndU, chronic inducible urticaria; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Table 7. Recommended diagnostic tests in frequent inducible urticaria subtypes (Adapted18). CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; UV, ultraviolet.
| *for identification of underlying causes or eliciting factors and for ruling out possible differential diagnoses if indicated. | ||
| Subtype | Routine Diagnostics Tests (Recommended) | Extended Diagnostic Program (Suggested Based on History Only)* |
| Cold urticaria | Cold provocation and threshold test (ice cube, cold water, cold wind) | Differential blood count and ESR or CRP, rule out other diseases, especially infections |
| Heat urticaria | Heat provocation and threshold test | None |
| Solar urticaria | UV and visible light of different wavelengths | Rule out other light-induced dermatoscs |
| Symptomatic dermographism | Elicit dermographism and threshold test (dermatographometer) | Differential blood count, ESR or CRP |
| Vibratory angioedema | Test with, for example, vortex | None |
| Aquagenic urticaria | Wet cloth at body temperature applied for 20 minutes | None |
| Cholinergic urticaria | Provocation testing | None |
| Contact urticaria | Cutaneous provocation test. Skin tests with immediate readings (e.g. prick test) |
None |
International guidelines
The need for international guidelines in urticaria
Clinical decisions regarding the best diagnostic and therapeutic approaches in patients with urticaria can vary from one allergist or dermatologist to another, and between clinical centres. International guidelines offer clear evidence-based recommendations that18:
- clarify which interventions are of proven benefit
- document the quality of the supporting data
- take into consideration regional differences in causative factors, medical systems and access to diagnosis and treatment worldwide
The 2017 International EAACI/GA2LEN/EDF/WAO Urticaria Guidelines
The only international recommendations for the diagnosis and management of urticaria are provided by the EAACI/GA²LEN/EDF/WAO urticaria guidelines18.
The 2017 International EAACI/GA2LEN/EDF/WAO urticaria guidelines are18:
- a joint initiative of four societies: the European Academy of Allergy and Clinical Immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF) and the World Allergy Organization (WAO)
- the result of a systematic literature review using a modified version of the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system and outcomes from a consensus conference on the 1 December 2016, with 44 urticaria experts from 25 countries
The 2017 revision and update to the international EAACI/GA2LEN/EDF/WAO urticaria guidelines include evidence-based recommendations for the definition, classification, diagnosis and management of urticaria18.
Table 8. Summary of the updated 2017 international EAACI/GA2LEN/EDF/WAO urticaria guidelines (Adapted18)
USA Guidelines
Recommendations for the diagnosis and management of urticaria in the USA are provided by the Joint Task Force on Practice Parameters (JTFPP) 2014 (Figure 41)202. The JTFPP is a 13-member task force consisting of representatives assigned by the American Academy of Allergy, Asthma and Immunology (AAAAI), the American College of Allergy, Asthma and Immunology (ACAAI) and the Joint Council of Allergy and Immunology.
The JTFPP urticaria guidelines treatment algorithm
Figure 41. The JTFPP urticaria guidelines treatment algorithm (Adapted18,202). LTRA, leukotriene receptor antagonist; NSAIDs, non-steroidal anti-inflammatory drugs.
UK Guidelines
Guidelines for the management of urticaria in the UK are provided by:
- The British Society for Allergy and Clinical Immunology (BSAI) 201577
- The British Association of Dermatologists (BAD) 2007203 – currently undergoing an update review
The National Institute for Health and Care Excellence (NICE) issued a technology appraisal guidance in previously untreated chronic spontaneous urticaria for omalizumab204.
Figure 42. The BSAI guidelines general management plan for chronic urticaria (Adapted77).
NICE technology appraisal guidance (TA33): Omalizumab for previously treated CSU204.
Omalizumab is recommended as an option as add-on therapy for treating severe CSU in adults and young people aged 12 years and over only if:
- the severity of the condition is assessed objectively, for example, using a weekly urticaria activity score of 28 or more
- the person's condition has not responded to standard treatment with H1-antihistamines and leukotriene receptor antagonists
- omalizumab is stopped at or before the fourth dose if the condition has not responded
- omalizumab is stopped at the end of a course of treatment (six doses) if the condition has responded, to establish whether the condition has gone into spontaneous remission, and is restarted only if the condition relapses. Omalizumab is administered under the management of a secondary care specialist in dermatology, immunology or allergy
- the company provides omalizumab with the discount agreed in the patient access scheme
People whose treatment with omalizumab is not recommended in this NICE guidance but was started within the National Health Service (NHS) before this guidance was published, should be able to continue treatment until they and their NHS clinician consider it appropriate to stop.
Regional guidelines
Figure 43. Regional and local guidelines for the diagnosis and management of urticaria (Adapted205–210).
Common to the majority of these guidelines are18,77,202–205,207–210:
- initial treatment with second generation H1-antihistamines at licensed doses
- escalation of dose of H1-antihistamine up to four-fold, or up to two-fold (Japan), if symptoms persist
- addition of other agents, e.g., omalizumab, ciclosporin or leukotriene receptor antagonists if an adequate response is still not achieved
Paediatric urticaria
Explore the available data in paediatric urticaria and discover its impact on younger patients, and how diagnosis and management compare to the recommendations for adult patients with CSU.
Epidemiology
Urticaria affects all age groups, however, acute urticaria appears more common in infants and young children while chronic urticaria appears more prevalent in adult patients. Interestingly, a Korean study published in 2018 found an unexpected peak in the prevalence of CU and CSU in subjects aged 0–9 years6. Overall, published data from paediatric patients remains scarce. In the 18-month Early Prevention of Asthma in Atopic Children (EPAAC) study involving 510 atopic children aged between 12 and 24 months, 42% of children receiving placebo experienced urticaria18,211. Outside of this atopic patient population, significant variation in the prevalence of acute and chronic urticaria has been observed (Figure 44).
Figure 44. Prevalence of paediatric urticaria (Adapted63,212–216)
Chronic urticaria is considered less prevalent in children than adults with a point prevalence of 0.1% to 0.3% identified in the UK212, although higher incidences have been observed in other studies215,217. The significant variation that has been observed within the published prevalence of paediatric urticaria around the world is likely to be influenced by different patient populations, clinical settings and diagnostic criteria. However, while the reported prevalence of urticaria within children has been variable, a number of consistencies have also been identified. Unlike the adult population where women are more likely to develop CSU than men, in the paediatric population urticaria appears to be distributed equally between both sexes214,218–220.
Although patient sex may not influence the incidence of paediatric urticaria, it does appear to change with age (Figure 45). A study consisting of 9,088 German new-borns from two prospective birth cohorts identified the incidence of urticaria as approximately 1% per year of age. However, the incidence for both sexes was highest during the second year of life and was typically higher in preschool children than in school-aged children214.
Figure 45. Incidence of urticaria by age and sex (Adapted214).
Aetiology: acute urticaria
The most common trigger for acute urticaria in children is infection. While these are typically viral infections of the upper respiratory tract, gastrointestinal and urinary infections, as well as bacterial and parasitic infections, have also been implicated221. The association between infection and acute urticaria is underlined by their seasonality with peaks in acute urticaria being observed to coincide with rises in viral respiratory infections222.
Other reported causes of acute urticaria in children include drug hypersensitivity and food allergy. However, detailed workups in two studies suggested that >90% of children with suspected drug hypersensitivity were able to tolerate the medication223,224. Meanwhile, food allergy was cited as the cause of urticaria in 2.7% and 6.3% of paediatric patients225,226.
Aetiology: chronic urticaria
Determining a trigger for paediatric chronic urticaria (spontaneous and inducible) can be difficult with reported identification rates ranging from 21% to 83%227.
While viral infection has been implicated as an exacerbating factor for chronic urticaria in children, it is not believed to play a causal role as has been indicated in acute urticaria228. Meanwhile, the role of bacterial infections in paediatric CSU remains uncertain with many studies suggesting it is unlikely to play a significant role227.
Like adult CSU, autoimmunity appears to be a critical factor in a significant number of patients. In fact, the frequency of autoimmune CSU appears to be similar between paediatric and adult patients227. In a Thai study of 94 paediatric CSU patients, 38% of patients were found to have a positive autologous serum skin test (ASST)229, while in an Italian study, 45% of children with CSU (22 of 49) were identified as having autoimmunity as the underlying cause230.
Chronic inducible urticaria is the most common chronic urticaria subtype in children with dermatographic urticaria and cholinergic urticaria being the two most frequently observed forms221,227. While cold urticaria is less common in paediatric patients, it is important to note that in one study of 30 patients, one-third of patients experienced anaphylactic reactions alongside their urticaria231.
Clinical presentation
As with adult CSU, hives and/or angioedema are the key symptoms in paediatric urticaria. However, the relative ratios of these symptoms at presentation remains less clear in younger patients. An early study indicated that 78.4% of paediatric patients experienced hives alone, while 6.6% suffered from angioedema alone and the remaining 15% had both232. A similar incidence of angioedema was observed in a prospective study that identified concomitant angioedema in 28% of CSU patients younger than 17 years of age220.
In contrast, a prospective study of 94 Thai children with CSU revealed that 51% had both hives and angioedema229 while a follow-up study from the same centre revealed 59.8% of children with chronic urticaria to suffer from hives and angioedema218. It is apparent that further research is required to establish the typical clinical presentation ratios within paediatric CSU.
Duration
The natural history of paediatric chronic urticaria remains uncertain. In one study, 25% of chronic urticaria patients went into remission during the three-year observation period233. In a more recent study, 18.5%, 54% and 72.1% of chronic urticaria patients were in remission 1, 3 and 5 years after the onset of symptoms218. Unsurprisingly, it appears that the underlying cause may influence remission rates. In a study of 139 patients with chronic urticaria under the age of 18, a resolution rate of 10.3 per 100 patient-years was observed. However, patients with a positive basophil activation test (BAT), indicative of autoimmune urticaria, were twice as likely to resolve after one year than those with negative BAT results (HR, 2.33; 95% CI, 1.08–5.05). Conversely, the presence of basophils decreased the likelihood of resolution (HR, 0.40; 95% CI, 0.20–0.99) (Figure 46)220.
Figure 46. Impact of BAT levels and presence of basophils on the probability of chronic urticaria resolution in patients under 18 years of age (Adapted220). BAT, basophil activation test.
Burden of disease
It is well established that CSU places a significant burden on adult patients’ quality of life5,24. However, it is becoming apparent that CSU also has an impact on children’s quality of life that is comparable or worse than many other chronic conditions (Figure 47)234.
Figure 47. Mean scores (± standard deviation) for Children’s Life Quality Index (CLQI) as given by 379 children with a chronic skin condition and 161 with other chronic diseases (Adapted234).
A meta-analysis that included the above Beattie study, used the Children’s Dermatology Life Quality Index (CDLQI) to investigate the quality of life impact of various skin conditions. It found urticaria to have a similar mean score (7.1) to psoriasis (8.0) and vitiligo (6.5) and a higher score than acne (5.3) and alopecia (3.1)235.
Educational impact of paediatric urticaria236:
- 7.4% of children missed school because of their condition-the average number of missed days was 7.5 ±18.5. In addition, 3.3% of parents had to take days off work because of their child’s urticaria
- children with chronic urticaria had significantly worse performance at school compared with children without urticaria (p=0.029). 4.8% of urticaria patients had “bad school performance” versus 1.9% in children without urticaria
Diagnosis
According to the EAACI/GA2LEN/EDF/WAO urticaria guidelines, the underlying causes of CSU in children are similar to those in adults and so the same diagnostic approach should be taken18.
In the diagnosis of urticaria in paediatric patients, targeted laboratory testing should be only used where appropriate217. The below table identifies what laboratory tests may be considered for different types of urticaria observed in paediatric patients (Table 9). Importantly, allergy tests must always be guided by patient history to avoid false positives. In addition, for patients with a suspected autoimmune cause for their urticaria, the B cell Histamine Release Assay is preferable to ASST due to the potential discomfort caused by the latter227.
Table 9. Relevant laboratory investigations for different types of urticaria in children. (Adapted217). ANA, antinuclear antibody; ASST, autologous serum skin test; CBC, complete blood count; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IgE, immunoglobulin E; TA, thyroid antibody; TFT, thyroid function test.
CSU severity is frequently assessed using the UAS7 score. A modified version of this scale has been used in paediatric patients to account for their smaller size and hence lower possible hive number. The modified UAS7 incorporates the following assessment over seven days237.
Hives (wheals)
0 = symptom is absent
1 = mild: (1–<10 hives/24 hour)
2 = moderate: (10–30 hives/24 h)
3 = intense: (>30 hives/24 h or large confluent areas of hives)
Pruritus
0 = symptom is absent
1 = mild: present but not annoying or troublesome
2 = moderate: troublesome, but does not interfere with normal daily activity or sleep
3 = intense: severe pruritus, which is sufficiently troublesome to interfere with normal activity or sleep
Management
With limited data available for the management of CSU in children, most management and treatment recommendations are based on an extrapolation of adult data.
It is strongly recommended that first-generation H1-antihistamines are not prescribed to infants and children with urticaria18
The initial step in managing paediatric urticaria is the identification of triggers, where possible, and their subsequent avoidance227. However, for many patients with urticaria, especially CSU, this is not possible. Pharmacological treatment remains symptomatic and paediatric patients over six months of age should initially be treated with second-generation H1-antihistamines18. These treatments have been shown to be very effective in a population of children with CSU (N=98; age = 0.5–16 years) with 50% achieving symptom control with licensed doses of second-generation H1-antihistamines (Figure 48). Furthermore, up to 96.9% of patients had controlled CSU symptoms when antihistamine doses were increased between 2 and 4 times the licensed dose219.
Figure 48. Percentage of patients achieving symptom control with licensed and increased doses of second-generation H1-antihistamines (Adapted219).
First-line therapy
The recommended first-line therapy for patients with CSU is second-generation H1-antihistamines. This also applies to paediatric and adolescent patients although the level of data available in this population means the guideline is a suggestion/conditional recommendation18.
The second-generation H1-antihistamines that have received approval for use in a paediatric/adolescent population are outlined in table 10. Despite the weak evidence for many of these treatments in child patients, randomised controlled trials have been completed in some cases and these are explored in more detail below.
Table 10. Licensed ages and doses for second-generation H1-antihistamines in children with urticaria (Adapted238,239,248,249,240–247). Please note, availability as well as licensed ages and doses may differ in your country. Please refer to local license information for more information.
Randomised controlled trials of first-line antihistamines in paediatric urticaria
The clinical data on the use of second-generation H1-antihistamines is sparse. However, limited data is available for some of the approved treatments.
Cetirizine
Cetirizine is approved in children two years of age and older and has been associated with minor effects on the CNS including somnolence, fatigue, dizziness and headache250.
Several clinical trials have been carried out to assess the efficacy of cetirizine in a range of different diseases including CSU251. A prospective, double-blind, parallel group study investigated how effective cetirizine was at preventing urticaria in 817 children (1–2 years) with atopic dermatitis over a period of 24 months. Acute urticaria was observed in 16.2% of children treated with placebo versus 5.8% treated with 0.25 mg/kg cetirizine252.
Desloratadine
A large post-marketing surveillance study of desloratadine has been performed in 9,246 German patients with CSU. While the study included patients as young as two years of age, the mean age was 43.2 years and no breakdown of the results by patient age is available189. However, the efficacy of desloratadine has been assessed in paediatric patients with CSU as a comparator arm in a study of rupatadine (see rupatadine section)237.
The safety of desloratadine syrup in paediatric patients with allergic rhinitis and acute urticaria has been assessed. Over a two-week period, 111 patients aged 2–5 years received 0.5 mg/ml desloratadine or placebo while 120 patients aged 6–11 years received 2.5 mg/ml desloratadine or placebo. In the study, no differences in the incidence of adverse events were observed between the desloratadine and placebo groups. Furthermore, there were no severe or serious adverse events reported, no clinically meaningful changes to median clinical laboratory tests and no significant changes in ECG results253. A short, 15-day course of desloratadine syrup or placebo was also studied in 255 children aged 6 months to 2 years who were candidates for, or receiving, antihistamine treatment for a range of atopic symptoms (such as itchy skin, sneezing and rhinorrhoea). In this patient population, desloratadine was observed to be well-tolerated and did not significantly influence vital signs or ECG results254.
Levocetirizine
Levocetirizine is the active enantiomer of cetirizine and although its efficacy at treating urticaria in children has not been assessed, its ability to prevent urticaria in atopic children has. In the Early Prevention of Asthma in Atopic Children Study, 510 children with atopic dermatitis who were aged between 1 and 2 years were given 0.125 mg/kg levocetirizine twice daily or matching placebo for 18 months. The incidence of urticaria was recorded in this patient population over the course of the study period (Figure 49)255.
Figure 49. Observed incidence of urticaria over an 18-month period in patients receiving levocetirizine (n/N = 70/255) or placebo (n/N = 106/255) (Adapted255).
The safety profile of levocetirizine was also assessed in the same study population. Over the 18-month study, similar rates of adverse events were observed in both groups and no significant differences were seen in each treatment groups’ height, mass, development or haematology and biochemistry tests255.
Loratadine
No clinical trials have assessed the efficacy of loratadine syrup in managing urticaria in paediatric patients. However, a double-blind, placebo-controlled, parallel-group study was performed to assess its tolerability in children aged 2–5 years with a history of allergic rhinitis or CSU. Over the course of 15 days, 5 mg of loratadine (n=60) or matching placebo (n=61) was given to children. Over this short timeframe, no differences were observed in the frequency of adverse events or ECG results between groups256.
Rupatadine
A double-blind, randomised, parallel-group, multicentre, placebo-controlled study compared the efficacy and tolerability of rupatadine in paediatric patients with CSU to placebo but also a desloratadine comparator treatment arm. In the study, 206 children aged 2–11 years received 1 mg/ml rupatadine, 0.5 mg/ml desloratadine or placebo once daily over a 6-week period.
The primary endpoint was the change observed in the modified UAS7 (Figure 50). Both rupatadine and desloratadine significantly reduced the observed UAS7 versus placebo over the treatment period.
Figure 50. Percentage change in modified UAS7 from baseline to the end of the 6-week treatment period by treatment arm (Adapted237). UAS7, 7-day Urticaria Activity Score.
Both rupatadine and desloratadine also significantly reduced the mean number of hives patients were experiencing versus placebo. However, only rupatadine significantly reduced the mean pruritus score versus placebo (Figure 51).
Figure 51. Percentage change in mean pruritus score from baseline to the end of the 6-week treatment period (Adapted)237.
Importantly, both rupatadine and desloratadine treatment resulted in a significant improvement in patients’ quality of life versus placebo as measured by the Children’s Dermatology Life Quality Index (CDLQI).
Finally, the safety profile of rupatadine and desloratadine were considered comparable to placebo. Only 10 observed adverse events were considered to be treatment-related with one of these in the rupatadine group (headache), five in the placebo group (decreased appetite, dizziness, headache, constipation, nausea) and four in the desloratadine group (blood alkaline phosphatase increase, abdominal pain, upper abdominal pain, constipation)237.
Second-line therapy
With little available data, the EAACI/GA2LEN/EDF/WAO guidelines recommend weight-adjusted up-dosing of second-generation H1-antihistamines as for adults18. While little clinical data is available, a study using a step wise increase in cetirizine, levocetirizine, desloratadine or fexofenadine dose did show increasing rates of response among children219.
However, alternative approaches have been suggested including addition of another second-generation H1-antihistamine, adding an H2-antagnoist, adding a leukotriene receptor antagonist or adding a first-generation H1-antihistamine at bedtime217. Little data exists for many of these solutions and most guidelines now strongly recommend against the use of first-generation antihistamines18.
Third-line therapy
Current guidelines recommend that paediatric patients follow the same treatment algorithm as adult patients. However, with little data available for many of these treatments in paediatric patients, the recommendation remains weak18. Here we explore the available data for recommended third-line therapies.
Ciclosporin
While ciclosporin is not licensed for the management of CSU, a trial as add-on therapy to antihistamines is recommended within the guidelines18.
A small study (N=7) of children aged between 9 and 16 years who remained symptomatic despite up-dosing of antihistamines, attempted treatment with 3 mg/kg/day divided twice daily. In these children, all experienced cessation of hives and were able to come off treatment. While no adverse events were reported, the authors recommend patients be carefully monitored for ciclosporin serum concentrations and renal function257.
Similar outcomes were observed in a more recent study that investigated ciclosporin treatment in 16 patients with CSU (aged 9–18 years) who were resistant to antihistamines258.
Despite the reported efficacy in these studies, the high incidence of adverse events reported in prior clinical trials suggest that ciclosporin should only be used in patients with severe disease that is refractory to antihistamines18.
Montelukast
Limited evidence suggests that montelukast may be effective for some patients with CSU who do not respond sufficiently to up-dosing of second-generation H1-antihistamines259. Further data have also suggested that it might be a useful add-on therapy before up-dosing of antihistamines260.
However, no clinical trials have evaluated the use of add-on montelukast in paediatric patients with CSU.
Omalizumab
The anti-IgE treatment omalizumab is recommended for the management of CSU patients who remain symptomatic despite up-dosing of second-generation H1-antihistamines18. It is licensed for patients ≥12 years of age and responses in patients aged between 12 and 17 years in Phase III clinical trials were comparable to the adult population261.
Comorbidities
With the likelihood of autoimmune diseases increasing with age, it is to be expected that children with CSU (<18 years) are less likely to have an autoimmune thyroid disease (AITD). Several studies have shown that the prevalence of IgG antithyroid autoantibodies are lower in children with CSU than adults (0.35%-1.6% vs. 4.2%-53.6%) as were the recorded rates of hypo- and hyperthyroidism. As a result, thyroid pathology does not appear to be an important factor for CSU in children94. However, a recent systematic review of available publications in children <12 years old with CSU identified a prevalence of anti-thyroid antibodies in 6.4% of patients262. More research is needed to understand the onset of AITD in younger patients with CSU.
While the prevalence of autoimmune diseases is lower among younger patients with CSU, that may not be the case for allergic comorbidities. An analysis of the omalizumab clinical trial programme identified 39 adolescents who had been enrolled in the programme. These patients were more likely to have experienced allergic rhinitis (61.5% vs. 43.2%) or asthma (56.4% vs. 22.3%) than adult patients, respectively263. An increased prevalence of allergic conditions has also been identified in children <12 years of age with CSU. In a systematic review, six publications including a total of 522 patients were identified and revealed a prevalence of atopy of 28.1%. This included 15.4% of patients reporting asthma, 13.8% reporting allergic rhinitis and 9.4% reporting atopic dermatitis262. However, controversy persists around the association between atopy and CSU in children. A 2017 study of 4,076 children in Korea found atopy to be significantly associated with acute urticaria but not chronic urticaria264.
References
- Fricke J, Ávila G, Keller T, Weller K, Lau S, Maurer M, et al. Prevalence of chronic urticaria in children and adults across the globe: Systematic review with meta-analysis. Allergy Eur J Allergy Clin Immunol. 2020;75(2):423–432.
- Watanabe J, Shimamoto J, Kotani K. The effects of antibiotics for helicobacter pylori eradication or dapsone on chronic spontaneous urticaria: A systematic review and meta-analysis. Antibiotics. 2021;10(2):1–14.
- NIH. What is Prevalence? https://www.nimh.nih.gov/health/statistics/what-is-prevalence. Accessed 5 July 2021.
- Alen Coutinho I, Regateiro FS, Fernandes RA, Pita JS, Gomes R, Coelho C, et al. Refractory chronic urticaria in adults: clinical characterization and predictors of severity. Allergy, Asthma Clin Immunol. 2020;16(1):1–9.
- Maurer M, Weller K, Bindslev-Jensen C, Giménez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy Eur J Allergy Clin Immunol. 2011;66(3):317–330.
- Kim BR, Yang S, Choi JW, Choi CW, Youn SW. Epidemiology and comorbidities of patients with chronic urticaria in Korea: A nationwide population-based study. J Dermatol. 2018;45(1):10–16.
- Ferrer M, Bartra J, Giménez-Arnau A, Jauregui I, Labrador-Horrillo M, Ortiz de Frutos J, et al. Management of urticaria: Not too complicated, not too simple. Clinical and Experimental Allergy. 2015;45(4):731–743.
- Lapi F, Cassano N, Pegoraro V, Cataldo N, Heiman F, Cricelli I, et al. Epidemiology of chronic spontaneous urticaria: Results from a nationwide, population-based study in Italy. Br J Dermatol. 2016;174(5):996–1004.
- Toubi E, Kessel A, Avshovich N, Bamberger E, Sabo E, Nusem D, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: A prospective study of 139 patients. Allergy Eur J Allergy Clin Immunol. 2004;59(8):869–873.
- Młynek A, Magerl M, Hanna M, Lhachimi S, Baiardini I, Canonica GW, et al. The German version of the chronic urticaria quality-of-life questionnaire: Factor analysis, validation, and initial clinical findings. Allergy Eur J Allergy Clin Immunol. 2009;64(6):927–936.
- Gattey N, Bahrani B, Hull PR. Chronic spontaneous urticaria: A questionnaire survey. J Cutan Med Surg. 2016;20(3):241–243.
- Beck LA, Bernstein JA, Maurer M. A review of international recommendations for the diagnosis and management of chronic urticaria. Acta Dermato-Venereologica. 2017;97(2):149–158.
- Curto-Barredo L, Riba Archilla L, Roura Vives G, Pujol RM, Giménez-Arnau AM. Clinical features of chronic spontaneous urticaria that predict disease prognosis and refractoriness to standard treatment. Acta Derm Venereol. 2018;98(7):641–647.
- Hellgren L. The Prevalence of Urticaria in the Total Population. Allergy. 1972;27(3):236–240.
- Kozel MMA, Mekkes JR, Bossuyt PMM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45(3):387–391.
- Staubach P, Onnen K, Vonend A, Metz M, Siebenhaar F, Tschentscher I, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: A placebo-controlled trial. Dermatology. 2006;212(2):150–159.
- Boonpiyathad T, Sangasapaviliya A. Autologous serum and plasma skin test to predict 2-year outcome in chronic spontaneous urticaria. Asia Pac Allergy. 2016;6(4):226–235.
- Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy Eur J Allergy Clin Immunol. 2018;73(7):1393–1414.
- Gaig P, Olona M, Muñoz Lejarazu D, Caballero MT, Domínguez FJ, Echechipia S, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14(3):214–220.
- Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: A representative cross-sectional population survey. Clin Exp Dermatol. 2010;35(8):869–873.
- Barniol C, Dehours E, Mallet J, Houze-Cerfon CH, Lauque D, Charpentier S. Levocetirizine and Prednisone Are Not Superior to Levocetirizine Alone for the Treatment of Acute Urticaria: A Randomized Double-Blind Clinical Trial. Ann Emerg Med. 2018;71(1):125-131.e1.
- Kathuria PC. Urticaria and its management. Indian J Allergy Asthma Immunol,. 2011;25(1):33–37.
- Karppinen A, Brummer-Korvenkontio H, Reunala T, Izquierdo I. Rupatadine 10 mg in the treatment of immediate mosquito-bite allergy. J Eur Acad Dermatology Venereol. 2012;26(7):919–922.
- Balp MM, Vietri J, Tian H, Isherwood G. The Impact of Chronic Urticaria from the Patient’s Perspective: A Survey in Five European Countries. Patient. 2015;8(6):551–558.
- Carne E. Managing chronic spontaneous urticaria (hives) in primary care. Nurs Stand. 2018;33(7):78–82.
- Gonçalo M, Gimenéz-Arnau A, Al-Ahmad M, Ben-Shoshan M, Bernstein JA, Ensina LF, et al. The global burden of chronic urticaria for the patient and society*. Br J Dermatol. 2021;184(2):226–236.
- Kang MJ, Kim HS, Kim HO, Park YM. The impact of chronic idiopathic urticaria on quality of life in Korean patients. Ann Dermatol. 2009;21(3):226–229.
- Silvares MRC, Fortes MRP, Miot HA. Quality of life in chronic urticaria: A survey at a public university outpatient clinic, Botucatu (Brazil). Rev Assoc Med Bras. 2011;57(5):565–569.
- Staubach P, Eckhardt-Henn A, Dechene M, Vonend A, Metz M, Magerl M, et al. Quality of life in patients with chronic urticaria is differentially impaired and determined by psychiatric comorbidity. Br J Dermatol. 2006;154(2):294–298.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy Eur J Allergy Clin Immunol. 2008;63(6):777–780.
- Engin B, Uguz F, Yilmaz E, Özdemir M, Mevlitoglu I. The levels of depression, anxiety and quality of life in patients with chronic idiopathic urticaria. J Eur Acad Dermatology Venereol. 2008;22(1):36–40.
- Staubach P, Dechene M, Metz M, Magerl M, Siebenhaar F, Weller K, et al. High prevalence of mental disorders and emotional distress in patients with chronic spontaneous urticaria. Acta Derm Venereol. 2011;91(5):557–561.
- Maurer M, Raap U, Staubach P, Richter-Huhn G, Bauer A, Oppel EM, et al. Antihistamine-resistant chronic spontaneous urticaria: 1-year data from the AWARE study. Clin Exp Allergy. 2019;49(5):655–662.
- Maurer M, Giménez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. Ligelizumab for Chronic Spontaneous Urticaria. N Engl J Med. 2019;381(14):1321–1332.
- Savic S, Leeman L, El-Shanawany T, Ellis R, Gach JE, Marinho S, et al. Chronic urticaria in the real-life clinical practice setting in the UK: results from the noninterventional multicentre AWARE study. Clin Exp Dermatol. 2020;45(8):1003–1010.
- Thomsen SF, Pritzier EC, Anderson CD, Vaugelade-Baust N, Dodge R, Dahlborn AK, et al. Chronic urticaria in the real-life clinical practice setting in Sweden, Norway and Denmark: baseline results from the non-interventional multicentre AWARE study. J Eur Acad Dermatology Venereol. 2017;31(6):1048–1055.
- Itakura A, Tani Y, Kaneko N, Hide M. Impact of chronic urticaria on quality of life and work in Japan: Results of a real-world study. J Dermatol. 2018;45(8):963–970.
- DeLong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: A cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol. 2008;144(1):35–39.
- Maurer M, Abuzakouk M, Bérard F, Canonica W, Oude Elberink H, Giménez-Arnau A, et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy Eur J Allergy Clin Immunol. 2017;72(12):2005–2016.
- Maurer M, Houghton K, Costa C, Dabove F, Ensina LF, Giménez-Arnau A, et al. Differences in chronic spontaneous urticaria between Europe and Central/South America: Results of the multi-center real world AWARE study. World Allergy Organ J. 2018;11(1):32.
- Bracken SJ, Abraham S, MacLeod AS. Autoimmune theories of chronic spontaneous urticaria. Frontiers in Immunology. 2019;10(MAR):627.
- Giménez-Arnau AM, DeMontojoye L, Asero R, Cugno M, Kulthanan K, Yanase Y, et al. The Pathogenesis of Chronic Spontaneous Urticaria: The Role of Infiltrating Cells. J Allergy Clin Immunol Pract. 2021;9(6):2195–2208.
- Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunological Reviews. 2018;282(1):232–247.
- Ferrer M. Immunological events in chronic spontaneous urticaria. Clinical and Translational Allergy. 2015;5(1). doi:10.1186/s13601-015-0074-7.
- Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. Journal of Allergy and Clinical Immunology. 2015;135(2):337-342.e2.
- Guillén-Aguinaga S, Jáuregui Presa I, Aguinaga-Ontoso E, Guillén-Grima F, Ferrer M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta-analysis. British Journal of Dermatology. 2016;175(6):1153–1165.
- Elias J, Boss E, Kaplan AP. Studies of the cellular infiltrate of chronic idiopathic urticaria: Prominence of T-lymphocytes, monocytes, and mast cells. J Allergy Clin Immunol. 1986;78(5 PART 1):914–918.
- Natbony SF, Phillips ME, Elias JM, Godfrey HP, Kaplan AP. Histologic studies of chronic idiopathic urticaria. J Allergy Clin Immunol. 1983;71(2):177–183.
- Sabroe RA, Poon E, Orchard GE, Lane D, Francis DM, Barr RM, et al. Cutaneous inflammatory cell infiltrate in chronic idiopathic urticaria: Comparison of patients with and without anti-FcεRI or anti-lgE autoantibodies. J Allergy Clin Immunol. 1999;103(3 II):484–493.
- Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: Comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109(4):694–700.
- Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy Eur J Allergy Clin Immunol. 2011;66(8):1107–1113.
- Sterba PM, Hamilton RG, Saini SS. Suppression of basophil FcεRI activation by serum from active chronic idiopathic/spontaneous urticaria (CIU/CSU) subjects. Journal of Investigative Dermatology. 2015;135(5):1454–1456.
- Metzger H, Alcaraz G, Hohman R, Kinet JP, Pribluda V, Quarto R. The receptor with high affinity for immunoglobulin E. Annual review of immunology. 1986;4:419–470.
- Ishizaka T, Conrad DH, Schulman ES, Sterk AR, Ishizaka K. Biochemical analysis of initial triggering events of IgE-mediated histamine release from human lung mast cells. J Immunol. 1983;130(5):2357–23562.
- Dehlink E, Fiebiger E. The Role of the High-Affinity IgE Receptor, FcϵRI, in Eosinophilic Gastrointestinal Diseases. Immunol Allergy Clin N Am. 2009;29(1):159–170.
- Rabe KF, Calhoun WJ, Smith N, Jimenez P. Can anti-IgE therapy prevent airway remodeling in allergic asthma? Allergy. 2011;66(9):1142–1151.
- Vonakis BM, Saini SS. New concepts in chronic urticaria. Current Opinion in Immunology. 2008;20(6):709–716.
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 SUPPL. 2):S73.
- Wernersson S, Pejler G. Mast cell secretory granules: Armed for battle. Nature Reviews Immunology. 2014;14(7):478–494.
- Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clinical and Experimental Allergy. 2009;39(6):777–787.
- Metz M, Maurer M. Omalizumab in chronic urticaria. Current Opinion in Allergy and Clinical Immunology. 2012;12(4):406–411.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105(4):664–672.
- Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. Ige mediated autoallergy against thyroid peroxidase - a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6(4):e14794.
- Staubach P, Vonend A, Burow G, Metz M, Magerl M, Maurer M. Patients with chronic urticaria exhibit increased rates of sensitisation to Candida albicans, but not to common moulds. Mycoses. 2009;52(4):334–338.
- Goh CL, Tan KT. Chronic autoimmune urticaria : WWhere we stand. In: Indian Journal of Dermatology. 2009. Wolters Kluwer Medknow Publications: 269–274.
- Jain S. Pathogenesis of chronic urticaria: An overview. Dermatology Research and Practice. 2014;2014. doi:10.1155/2014/674709.
- Siebenhaar F, Melde A, Magerl M, Zuberbier T, Church MK, Maurer M. Histamine intolerance in patients with chronic spontaneous urticaria. J Eur Acad Dermatology Venereol. 2016;30(10):1774–1777.
- O’Donnell B, Lawlor F, Simpson J, Morgan M, Greaves M. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136(2):197–201.
- Barbosa F, Freitas J, Barbosa A. Chronic idiopathic urticaria and anxiety symptoms. J Health Psychol. 2011;16(7):1038–1047.
- Grattan C. The urticarias: Pathophysiology and management. Clin Med J R Coll Physicians London. 2012;12(2):164–167.
- Kulthanan K, Jiamton S, Boochangkool K, Jongjarearnprasert K. Angioedema: Clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
- Weller K, Maurer M, Grattan C, Nakonechna A, Abuzakouk M, Bérard F, et al. ASSURE-CSU: A real-world study of burden of disease in patients with symptomatic chronic spontaneous urticaria. Clin Transl Allergy. 2015;5(1). doi:10.1186/s13601-015-0072-9.
- Stull DE, McBride D, Houghton K, Finlay AY, Gnanasakthy A, Balp MM. Assessing Changes in Chronic Spontaneous/Idiopathic Urticaria: Comparisons of Patient-Reported Outcomes Using Latent Growth Modeling. Adv Ther. 2016;33(2):214–224.
- Goldstein S, Eftekhari S, Mitchell L, Winders TA, Kaufman L, Dudas D, et al. Perspectives on living with chronic spontaneous urticaria: From onset through diagnosis and disease management in the US. Acta Derm Venereol. 2019;99(12):1091–1098.
- Cappuccio A, Limonta T, Parodi A, Cristaudo A, Bugliaro F, Cannavò SP, et al. Living with chronic spontaneous urticaria in Italy: A narrative medicine project to improve the pathway of patient care. Acta Derm Venereol. 2017;97(1):81–85.
- Powell RJ, Leech SC, Till S, Huber PAJ, Nasser SM, Clark AT. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45(3):547–565.
- Magerl M, Altrichter S, Borzova E, Giménez-Arnau A, Grattan CEH, Lawlor F, et al. The definition, diagnostic testing, and management of chronic inducible urticarias - The EAACI/GA2LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy Eur J Allergy Clin Immunol. 2016;71(6):780–802.
- Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA2LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: The 2013 revision and update. Allergy Eur J Allergy Clin Immunol. 2014;69(7):868–887.
- Bansal CJ, Bansal AS. Stress, pseudoallergens, autoimmunity, infection and inflammation in chronic spontaneous urticaria. Allergy, Asthma and Clinical Immunology. 2019;15(1):56.
- Kumaran M, Mangal S, Narang T, Parsad D. Autologous serum and plasma skin tests in chronic spontaneous urticaria: A reappraisal. Indian Dermatol Online J. 2017;8(2):94.
- Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CEH. EAACI/GA2LEN task force consensus report: The autologous serum skin test in urticaria. Allergy Eur J Allergy Clin Immunol. 2009;64(9):1256–1268.
- Weller K, Groffik A, Magerl M, Tohme N, Martus P, Krause K, et al. Development, validation, and initial results of the Angioedema Activity Score. Allergy Eur J Allergy Clin Immunol. 2013;68(9):1185–1192.
- Baiardini I, Pasquali M, Braido F, Fumagalli F, Guerra L, Compalati E, et al. A new tool to evaluate the impact of chronic urticaria on quality of life: Chronic urticaria quality of life questionnaire (CU-Q2oL). Allergy Eur J Allergy Clin Immunol. 2005;60(8):1073–1078.
- Weller K, Groffik A, Magerl M, Tohme N, Martus P, Krause K, et al. Development and construct validation of the angioedema quality of life questionnaire. Allergy Eur J Allergy Clin Immunol. 2012;67(10):1289–1298.
- Hollis K, Proctor C, McBride D, Balp MM, McLeod L, Hunter S, et al. Comparison of Urticaria Activity Score Over 7 Days (UAS7) Values Obtained from Once-Daily and Twice-Daily Versions: Results from the ASSURE-CSU Study. Am J Clin Dermatol. 2018;19(2):267–274.
- Stull D, McBride D, Tian H, Gimenez Arnau A, Maurer M, Marsland A, et al. Analysis of disease activity categories in chronic spontaneous/idiopathic urticaria. Br J Dermatol. 2017;177(4):1093–1101.
- Hawro T, Saluja R, Weller K, Altrichter S, Metz M, Maurer M. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy. 2014;69(1):113–117.
- Baiardini I, Braido F, Bindslev-Jensen C, Bousquet PJ, Brzoza Z, Canonica GW, et al. Recommendations for assessing patient-reported outcomes and health-related quality of life in patients with urticaria: A GA2LEN taskforce position paper. Allergy Eur J Allergy Clin Immunol. 2011;66(7):840–844.
- Kocatürk E, Can PK, Akbas PE, Copur M, Degirmentepe EN, Kızıltac K, et al. Management of chronic inducible urticaria according to the guidelines: A prospective controlled study. J Dermatol Sci. 2017;87(1):60–69.
- Ring J. Chronic urticaria: New hope for an old disease. Journal of the European Academy of Dermatology and Venereology. 2016;30:3–4.
- Tat TS. Higher levels of depression and anxiety in patients with chronic urticaria. Med Sci Monit. 2019;25:115–120.
- Maurer M, Magerl M, Metz M, Zuberbier T. Revisions to the international guidelines on the diagnosis and therapy of chronic urticaria. JDDG - J Ger Soc Dermatology. 2013;11(10):971–978.
- Kolkhir P, Metz M, Altrichter S, Maurer M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: A systematic review. Allergy Eur J Allergy Clini Immunol. 2017;72(10):1440–1460.
- Kolkhir P, Borzova E, Grattan C, Asero R, Pogorelov D, Maurer M. Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun Rev. 2017;16(12):1196–1208.
- Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: Associations found in a large population study. J Allergy Clin Immunol. 2012;129(5):1307–1313.
- Magen E, Chikovani T, Waitman DA, Kahan NR. Association of alopecia areata with atopic dermatitis and chronic spontaneous urticaria. Allergy Asthma Proc. 2018;39(2):96–102.
- Shalom G, Magen E, Dreiher J, Freud T, Bogen B, Comaneshter D, et al. Chronic urticaria and atopic disorders: a cross-sectional study of 11 271 patients. Br J Dermatol. 2017;177(4):e96–e97.
- Ban GY, Kim MY, Yoo HS, Nahm DH, Ye YM, Shin YS, et al. Clinical features of elderly chronic urticaria. Korean J Intern Med. 2014;29(6):800–806.
- Bewley A. WORKING PARTY REPORT ON MINIMUM STANDARDS FOR PSYCHODERMATOLOGY SERVICES 2012 Members of the Working Party Accessing Psychodermatology services. 2012 https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=1622. Accessed 7 May 2021.
- Konstantinou GN, Konstantinou GN. Psychiatric comorbidity in chronic urticaria patients: A systematic review and meta-analysis. Clinical and Translational Allergy. 2019;9(1):1–12.
- Conrad R, Geiser F, Haidl G, Hutmacher M, Liedtke R, Wermter F. Relationship between anger and pruritus perception in patients with chronic idiopathic urticaria and psoriasis. J Eur Acad Dermatol Venereol. 2008;22(9):1062–1069.
- Chung MC, Symons C, Gilliam J, Kaminski ER. The relationship between posttraumatic stress disorder, psychiatric comorbidity, and personality traits among patients with chronic idiopathic urticaria. Compr Psychiatry. 2010;51(1):55–63.
- Gupta MA, Gupta AK. Chronic idiopathic urticaria and post-traumatic stress disorder (PTSD): An under-recognized comorbidity. Clinics in Dermatology. 2012;30(3):351–354.
- Gupta MA, Jarosz P, Gupta AK. Posttraumatic stress disorder (PTSD) and the dermatology patient. Clin Dermatol. 2017;35(3):260–266.
- Mendelson MH, Bernstein JA, Gabriel S, Balp MM, Tian H, Vietri J, et al. Patient-reported impact of chronic urticaria compared with psoriasis in theUnited States. J Dermatolog Treat. 2017;28(3):229–236.
- Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2015;56(5):668–74.
- Maurer M, Church MK, Gonçalo M, Sussman G, Sánchez-Borges M. Management and treatment of chronic urticaria (CU). J Eur Acad Dermatology Venereol. 2015;29(S3):16–32.
- Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, et al. EAACI/GALEN/EDF/WAO guideline: Management of urticaria. Allergy Eur J Allergy Clin Immunol. 2009;64(10):1427–1443.
- Church MK, Maurer M, Simons FER, Bindslev-Jensen C, Van Cauwenberge P, Bousquet J, et al. Risk of first-generation H1-antihistamines: A GA2LEN position paper. Allergy. 2010;65(4):459–466.
- Simon FER, Simons KJ. H1 Antihistamines: Current Status and Future Directions. World Allergy Organ J. 2008;1(9):145–155.
- Phinyo P, Koompawichit P, Nochaiwong S, Tovanabutra N, Chiewchanvit S, Chuamanochan M. Comparative Efficacy and Acceptability of Licensed Dose Second-Generation Antihistamines in Chronic Spontaneous Urticaria: A Network Meta-Analysis. J Allergy Clin Immunol Pract. 2021;9(2):956-970.e57.
- Staevska M, Gugutkova M, Lazarova C, Kralimarkova T, Dimitrov V, Zuberbier T, et al. Night-time sedating H1-antihistamine increases daytime somnolence but not treatment efficacy in chronic spontaneous urticaria: A randomized controlled trial. Br J Dermatol. 2014;171(1):148–154.
- Zuberbier T, Oanta A, Bogacka E, Medina I, Wesel F, Uhl P, et al. Comparison of the efficacy and safety of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: A multi-centre, double-blind, randomized, placebo-controlled study. Allergy Eur J Allergy Clin Immunol. 2010;65(4):516–528.
- Yagami A, Furue M, Togawa M, Saito A, Hide M. One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases. J Dermatol. 2017;44(4):375–385.
- Juhlin L, Arendt C. Treatment of chronic urticaria with cetirizine dihydrochloride a non‐sedating antihistamine. Br J Dermatol. 1988;119(1):67–72.
- Kalivas J, Breneman D, Tharp M, Bruce S, Bigby M, nine other investigators. Urticaria: Clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy Clin Immunol. 1990;86(6 PART 2):1014–1018.
- Dakhale GN, Shinde AT, Mahatme MS, Hiware SK, Mishra DB, Mukhi JI, et al. Clinical effectiveness and safety of cetirizine versus rupatadine in chronic spontaneous urticaria: A randomized, double-blind, 6-week trial. Int J Dermatol. 2014;53(5):643–649.
- Ring J, Hein R, Gauger A, Bronsky E, Miller B. Once-daily desloratadine improves the signs and symptoms of chronic idiopathic urticaria: A randomized, double-blind, placebo-controlled study. Int J Dermatol. 2001;40(1):72–76.
- Monroe E, Finn A, Patel P, Guerrero R, Ratner P, Bernstein D, et al. Efficacy and safety of desloratadine 5 mg once daily in the treatment of chronic idiopathic urticaria: A double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol. 2003;48(4):535–541.
- Ortonne JP, Grob JJ, Auquier P, Dreyfus I. Efficacy and safety of desloratadine in adults with chronic idiopathic urticaria: A randomized, double-blind, placebo-controlled, multicenter trial. Am J Clin Dermatol. 2007;8(1):37–42.
- Lachapelle JM, Decroix J, Henrijean A, Roquet-Gravy PP, De Swerdt A, Boonen H, et al. Desloratadine 5 mg once daily improves the quality of life of patients with chronic idiopathic urticaria. J Eur Acad Dermatology Venereol. 2006;20(3):288–292.
- Grob JJ, Auquier P, Dreyfus I, Ortonne JP. How to prescribe antihistamines for chronic idiopathic urticaria: Desloratadine daily vs PRN and quality of life. Allergy Eur J Allergy Clin Immunol. 2009;64(4):605–612.
- Weller K, Ardelean E, Scholz E, Martus P, Zuberbier T, Maurer M. Can on-demand non-sedating antihistamines improve Urticaria symptoms? A double-blind, randomized, single-dose study. Acta Derm Venereol. 2013;93(2):168–174.
- Potter PC, Kapp A, Maurer M, Guillet G, Jian AM, Hauptmann P, et al. Comparison of the efficacy of levocetirizine 5 mg and desloratadine 5 mg in chronic idiopathic urticaria patients. Allergy Eur J Allergy Clin Immunol. 2009;64(4):596–604.
- Hong JB, Lee HC, Hu FC, Chu CY. A randomized, double-blind, active-controlled, parallel-group pilot study to compare the efficacy and sedative effects of desloratadine 5 mg with levocetirizine 5 mg in the treatment of chronic idiopathic urticaria. J Am Acad Dermatol. 2010;63(5):e100–e102.
- Kolasani BP, Mudium R, Reddy N. A comparative study of efficacy and safety of rupatadine versus desloratadine in patientswith chronic idiopathic urticaria. Asian J Biomed Pharm Sci. 2013;3(21):42–47.
- Goyal V, Gupta A, Gupta O, Lal D, Gill M. Comparative efficacy and safety of ebastine 20 mg, ebastine 10 mg and levocetirizine 5 mg in acute urticaria. J Clin Diagnostic Res. 2017;11(3):WC06–WC09.
- Kalis B. Double-blind multicentre comparative study of ebastine, terfenadine and placebo in the treatment of chronic idiopathic urticaria in adults. Drugs. 1996;52(SUPPL. 1):30–34.
- Josefson D. Hay fever drug to be banned by the FDA. BMJ. 1997;314(7076):248.
- Finn AF, Kaplan AP, Fretwell R, Qu R, Long J. A double-blind, placebo-controlled trial of fexofenadine HCl in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1999;104(5):1071–1078.
- Nelson HS, Reynolds R, Mason J. Fexofenadine HCl is safe and effective for treatment of chronic idiopathic urticaria. Ann Allergy, Asthma Immunol. 2000;84(5):517–522.
- Kulthanan K, Sitakalin C, Korkij W, Janjumratsang P, Kuntiranont M, Gherunpong N, et al. Multicenter study of the efficacy and safety of fexofenadine 60 mg. Twice daily in 108 Thai patients with chronic idiopathic urticaria. J Med Assoc Thail. 2001;84(2):153–159.
- Thompson AK, Finn AF, Schoenwetter WF. Effect of 60 mg twice-daily fexofenadine HCL on quality of life, work and classroom productivity, and regular activity in patients with chronic idiopathic urticaria. J Am Acad Dermatol. 2000;43(1 I):24–30.
- Characteristics P. Fexofenadine hydrochloride 180mg film-coated Tablets. 2020;3–7.
- Kaplan AP, Spector SL, Meeves S, Liao Y, Varghese ST, Georges G. Once-daily fexofenadine treatment for chronic idiopathic urticaria: A multicenter, randomized, double-blind, placebo-controlled study. Ann Allergy, Asthma Immunol. 2005;94(6):662–669.
- Spector SL, Shikiar R, Harding G, Meeves S, Leahy MJ. The effect of fexofenadine hydrochloride on productivity and quality of life in patients with chronic idiopathic urticaria. Cutis. 2007;79(2):157–162.
- Handa S, Dogra S, Kumar B. Comparative efficacy of cetirizine and fexofenadine in the treatment of chronic idiopathic urticaria. J Dermatolog Treat. 2004;15(1):55–57.
- Dhar S, Varghese ST, Georges G. Assessing the therapeutic benefits of antihistamines for the treatment of chronic idiopathic urticaria - A requirement for well-designed comparative clinical studies. J Dermatol Treat. 2005;16(3):176–177.
- Nettis E, Colanardi MC, Barra L, Ferrannini A, Vacca A, Tursi A. Levocetirizine in the treatment of chronic idiopathic urticaria: A randomized, double-blind, placebo-controlled study. Br J Dermatol. 2006;154(3):533–538.
- Kapp A, Pichler WJ. Levocetirizine is an effective treatment in patients suffering from chronic idiopathic urticaria: A randomized, double-blind, placebo-controlled, parallel, multicenter study. Int J Dermatol. 2006;45(4):469–474.
- Garg G, Thami GP. Comparative efficacy of cetirizine and levocetirizine in chronic idiopathic urticaria. J Dermatolog Treat. 2007;18(1):23–24.
- Podder I, Das A, Ghosh S, Biswas D, Sengupta S, Chowdhury SN. Effectiveness, safety, and tolerability of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic spontaneous urticaria: A double-blind, parallel group, randomized controlled trial. Dermatol Ther. 2020;33(6). doi:10.1111/dth.13946.
- Anuradha P, Maiti R, Jyothirmai J, Mujeebuddin O, Anuradha M. Loratadine versus levocetirizine in chronic idiopathic urticaria: A comparative study of efficacy and safety. Indian J Pharmacol. 2010;42(1):12–16.
- Maiti R, Rahman J, Jaida J, Allala U, Palani A. Rupatadine and levocetirizine for seasonal allergic rhinitis: A comparative study of efficacy and safety. Arch Otolaryngol - Head Neck Surg. 2010;136(8):796–800.
- Ventegodt S. World health organization model list of essential medicines. 2015 https://www.who.int/medicines/publications/essentialmedicines/en/. Accessed 6 March 2020.
- ABU SHAREEAH AM. Comparative Eficacy of Loratadine and Terfenadine in the Treatment of Chronic Idiopathic Urticaria. Int J Dermatol. 1992;31(5):355–356.
- Monroe EW, Bernstein DI, Fox RW, Grabiec S V., Honsinger RW, Kalivas JT, et al. Relative efficacy and safety of loratadine, hydroxyzine, and placebo in chronic idiopathic urticaria. Arzneimittel-Forschung/Drug Res. 1992;42(9):1119–1121.
- Metz M, Maurer M. Rupatadine for the treatment of allergic rhinitis and urticaria. Expert Rev Clin Immunol. 2011;7(1):15–20.
- Nettis E, Delle Donne P, Di Leo E, Calogiuri GF, Ferrannini A, Vacca A. Rupatadine for the treatment of urticaria. Expert Opin Pharmacother. 2013;14(13):1807–1813.
- Gimenez-Arnau A, Pujol RM, Ianosi S, Kaszuba A, Malbran A, Poop G, et al. Rupatadine in the treatment of chronic idiopathic urticaria: A double-blind, randomized, placebo-controlled multicentre study. Allergy Eur J Allergy Clin Immunol. 2007;62(5):539–546.
- González-Núñez V, Bachert C, Mullol J. Rupatadine: global safety evaluation in allergic rhinitis and urticaria. Expert Opin Drug Saf. 2016;15(10):1439–1448.
- Hide M, Suzuki T, Tanaka A, Aoki H. Efficacy and safety of rupatadine in Japanese adult and adolescent patients with chronic spontaneous urticaria: A double-blind, randomized, multicenter, placebo-controlled clinical trial. Allergol Int. 2019;68(1):59–67.
- Metz M, Weller K, Neumeister C, Izquierdo I, Bödeker RH, Schwantes U, et al. Rupatadine in Established Treatment Schemes Improves Chronic Spontaneous Urticaria Symptoms and Patients’ Quality of Life: a Prospective, Non-interventional Trial. Dermatol Ther (Heidelb). 2015;5(4):217–230.
- Krause K, Spohr A, Zuberbier T, Church MK, Maurer M. Up-dosing with bilastine results in improved effectiveness in cold contact urticaria. Allergy Eur J Allergy Clin Immunol. 2013;68(7):921–928.
- Simons FER. Advances in H 1 -Antihistamines . N Engl J Med. 2004;351(21):2203–2217.
- Holgate ST, Canonica GW, F. Estelle R. Simons, Taglialatela M, Tharp M, Timmerman H, et al. Consensus group on new-generation antihistamines (CONGA): Present status and recommendations. Clin Exp Allergy. 2003;33(9):1305–1324.
- Hindmarch I, Johnson S, Meadows R, Kirkpatrick T, Shamsi Z. The acute and sub-chronic effects of levocetirizine, cetirizine, loratadine, promethazine and placebo on cognitive function, psychomotor performance, and weal and flare. Curr Med Res Opin. 2001;17(4):241–255.
- Shamsi Z, Hindmarch I. Sedation and antihistamines: A review of inter-drug differences using proportional impairment ratios. Human Psychopharmacol. 2000;15(SUPPL. 1):S3–S30.
- Casale TB, Blaiss MS, Gelfand E, Gilmore T, Harvey PD, Hindmarch I, et al. First do no harm: Managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003;111(5):S835–S842.
- Van den Elzen MT, Van Os-Medendorp H, Van den Brink I, Van den Hurk K, Kouznetsova OI, Lokin ASHJ, et al. Effectiveness and safety of antihistamines up to fourfold or higher in treatment of chronic spontaneous urticaria. Clin Transl Allergy. 2017;7(1):4.
- Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H 1-antihistamine up-dosing in chronic spontaneous urticaria: Patients’ perspective of effectiveness and side effects - a retrospective survey study. PLoS One. 2011;6(9):e23931.
- Sánchez-Borges M, Ansotegui I, Jimenez JM, Rojo MI, Serrano C, Yañez A. Comparative efficacy of non-sedating antihistamine updosing in patients with chronic urticaria. World Allergy Organ J. 2014;7(1):33.
- Iriarte Sotés P, Armisén M, Usero-Bárcena T, Rodriguez Fernández A, Otero Rivas MM, Gonzalez MT, et al. Efficacy and safety of up-dosing antihistamines in chronic spontaneous urticaria: A systematic review of the literature. J Investig Allergol Clin Immunol. 2021;31(4):282–291.
- Asero R. Chronic unremitting urticaria: Is the use of antihistamines above the licensed dose effective? A preliminary study of cetirizine at licensed and above-licensed doses. Clin Exp Dermatol. 2007;32(1):34–38.
- Kameyoshi Y, Tanaka T, Mihara S, Takahagi S, Niimi N, Hide M. Increasing the dose of cetirizine may lead to better control of chronic idiopathic urticaria: An open study of 21 patients [3]. Bri J Dermatol. 2007;157(4):803–804.
- Staevska M, Popov TA, Kralimarkova T, Lazarova C, Kraeva S, Popova D, et al. The effectiveness of levocetirizine and desloratadine in up to 4 times conventional doses in difficult-to-treat urticaria. J Allergy Clin Immunol. 2010;125(3):676–682.
- Dubertret L, Zalupca L, Cristodoulo T, Benea V, Medina I, Fantin S, et al. Once-daily rupatadine improves the symptoms of chronic idiopathic urticaria: A randomised, double-blind, placebo-controlled study. Eur J Dermatology. 2007;17(3):223–228.
- Giménez-Arnau A, Izquierdo I, Maurer M. The use of a responder analysis to identify clinically meaningful differences in chronic urticaria patients following placebo- controlled treatment with rupatadine 10 and 20 mg. J Eur Acad Dermatology Venereol. 2009;23(9):1088–1091.
- EMA. Xolair Summary of Opinion. 2014. https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-xolair_en.pdf. Accessed 7 May 2021.
- FDA. Highlights of prescribing information. www.fda.gov/medwatch. Accessed 7 May 2021.
- Maurer M, Rosén K, Hsieh H-J, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the Treatment of Chronic Idiopathic or Spontaneous Urticaria. N Engl J Med. 2013;368(10):924–935.
- Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bülbül Baskan E, Bradley MS, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on h 1 antihistamines: A randomized, placebo-controlled study. J Invest Dermatol. 2015;135(1):67–75.
- Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132(1):101–109.
- Stull D, McBride D, Georgiou P, Zuberbier T, Grattan C BM-M. Measuring patient severity in CSU as categorical health states: efficient and informative? EAACI Congr. 2014;69(Suppl s99):317.
- Casale T, Maurer M, Saini SS, Bernstein J. Omalizumab reduced symptoms and improved health-related quality of life (HRQoL) in patients with refractory chronic spontaneous/idiopathic urticaria (CSU/CIU) in three randomized, doubleblind, placebo-controlled phase III trials: a post-hoc analysis of pe. In: Allergy. 2014: 573–619.
- Bérard F, Ferrier Le Bouedec MC, Bouillet L, Reguiai Z, Barbaud A, Cambazard F, et al. Omalizumab in patients with chronic spontaneous urticaria nonresponsive to H1-antihistamine treatment: results of the phase IV open-label SUNRISE study. Br J Dermatol. 2019;180(1):56–66.
- Casale TB, Win PH, Bernstein JA, Rosén K, Holden M, Iqbal A, et al. Omalizumab response in patients with chronic idiopathic urticaria: Insights from the XTEND-CIU study. J Am Acad Dermatol. 2018;78(4):793–795.
- Agache I, Rocha C, Pereira A, Song Y, Alonso-Coello P, Solà I, et al. Efficacy and safety of treatment with omalizumab for chronic spontaneous urticaria: A systematic review for the EAACI Biologicals Guidelines. Allergy Eur J Allergy Clin Immunol. 2021;76(1):59–70.
- Deza G, Bertolín-Colilla M, Pujol RM, Curto-Barredo L, Soto D, García M, et al. Basophil Fc3 RI expression in chronic spontaneous urticaria: A potential immunological predictor of response to omalizumab therapy. Acta Derm Venereol. 2017;97(6):698–704.
- Jörg L, Pecaric-Petkovic T, Reichenbach S, Coslovsky M, Stalder O, Pichler W, et al. Double-blind placebo-controlled trial of the effect of omalizumab on basophils in chronic urticaria patients. Clin Exp Allergy. 2018;48(2):196–204.
- Marzano A V., Genovese G, Casazza G, Fierro MT, Dapavo P, Crimi N, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatology Venereol. 2019;33(5):918–924.
- Magen E, Chikovani T, Waitman DA, Kahan NR. Factors related to omalizumab resistance in chronic spontaneous urticaria. Allergy Asthma Proc. 2019;40(4):273–278.
- Asero R, Tedeschi A, Cugno M. Treatment of refractory chronic urticaria: Current and future therapeutic options. American Journal of Clinical Dermatology. 2013;14(6):481–488.
- Kulthanan K, Subchookul C, Hunnangkul S, Chularojanamontri L, Tuchinda P. Factors predicting the response to cyclosporin treatment in patients with chronic spontaneous urticaria: A systematic review. Allergy, Asthma and Immunology Research. 2019;11(5):736–755.
- Savic S, Marsland A, McKay D, Ardern-Jones MR, Leslie T, Somenzi O, et al. Retrospective case note review of chronic spontaneous urticaria outcomes and adverse effects in patients treated with omalizumab or ciclosporin in UK secondary care. Allergy, Asthma Clin Immunol. 2015;11(1):21.
- Asero R, Tedesch A. Usefulness of a short course of oral prednisone in antihistamine-resistant chronic urticaria: A retrospective analysis. J Investig Allergol Clin Immunol. 2010;20(5):386–390.
- Weller K, Church MK, Hawro T, Altrichter S, Labeaga L, Magerl M, et al. Updosing of bilastine is effective in moderate to severe chronic spontaneous urticaria: A real-life study. Allergy. 2018;73(10):2073–2075.
- Augustin M, Ehrle S. Safety and efficacy of desloratadine in chronic idiopathic urticaria in clinical practice: An observational study of 9246 patients. J Eur Acad Dermatology Venereol. 2009;23(3):292–299.
- Bachert C, Maurer M. Safety and efficacy of desloratadine in subjects with seasonal allergic rhinitis or chronic urticaria: Results of four postmarketing surveillance studies. Clin Drug Investig. 2010;30(2):109–122.
- Yin OQP, Shi XJ, Tomlinson B, Chow MSS. Effect of CYP2D6*10 allele on the pharmacokinetics of loratadine in Chinese subjects. Drug Metab Dispos. 2005;33(9):1283–1287.
- Fang SY, Perng DW, Lee JYY, Lin DY, Huang CY. An open-label, multicentre study of levocetirizine for the treatment of allergic rhinitis and Urticaria in Taiwanese Patients. Chin J Physiol. 2010;53(4):199–207.
- Of H, Of H, Information P, Information P. Highlights of prescribing information. Metabolism Clinical And Experimental. 2008. https://www.gene.com/download/pdf/xolair_prescribing.pdf. Accessed 7 May 2021.
- Novartis Pharmaceuticals Australia Pty Ltd. Xolair (Omalizumab) [Bula]. 2014;1–31.
- Eghrari-Sabet J, Sher E, Kavati A, Pilon D, Zhdanava M, Balp MM, et al. Real-world use of omalizumab in patients with chronic Idiopathic/Spontaneous urticaria in the United States. Allergy Asthma Proc. 2018;39(3):191–200.
- Wang Z, Li M, Wang Y, Xu D, Wang Q, Zhang S, et al. Long-term mortality and morbidity of patients with systemic lupus erythematosus: a single-center cohort study in China. Lupus. 2018;27(5):864–869.
- Wang L, Ke X, Kavati A, Wertz D, Huang Q, Willey VJ, et al. Real-world treatment patterns and outcomes of omalizumab use in patients with chronic idiopathic urticaria. Curr Med Res Opin. 2018;34(1):35–39.
- Syrigos N, Grapsa D, Zande M, Tziotou M, Syrigou E. Treatment response to omalizumab in patients with refractory chronic spontaneous urticaria. Int J Dermatol. 2018;57(4):417–422.
- Tharp MD, Bernstein JA, Kavati A, Ortiz B, MacDonald K, Denhaerynck K, et al. Benefits and Harms of Omalizumab Treatment in Adolescent and Adult Patients with Chronic Idiopathic (Spontaneous) Urticaria: A Meta-analysis of ‘real-world’ Evidence. JAMA Dermatology. 2019;155(1):29–38.
- Metz M, Vadasz Z, Kocatürk E, Giménez-Arnau AM. Omalizumab Updosing in Chronic Spontaneous Urticaria: an Overview of Real-World Evidence. Clin Rev Allergy Immunol. 2020;59(1):38–45.
- Maurer M, Giménez-Arnau A, Ensina LF, Chu CY, Jaumont X, Tassinari P. Chronic urticaria treatment patterns and changes in quality of life: AWARE study 2-year results. World Allergy Organ J. 2020;13(9):100460.
- Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270-1277.e66.
- Grattan CEH, Humphreys F. Guidelines for evaluation and management of urticaria in adults and children. Br J Dermatol. 2007;157(6):1116–1123.
- National Institute for Health and Care Excellence (NICE). Omalizumab for previously treated chronic spontaneous urticaria - NICE technology Guidance 339. 2015;1–56.
- Hide M, Hiragun T. Japanese guidelines for diagnosis and treatment of urticaria in comparison with other countries. Allergol Int. 2012;61(4):517–527.
- Tedeschi A, Girolomoni G, Asero R. AAITO position paper. Chronic urticaria: Diagnostic workup and treatment. Eur Ann Allergy Clin Immunol. 2007;39(7):225–231.
- Godse K, Rajagopalan M, Girdhar M, Kandhari S, Shah B, Chhajed P, et al. Position statement for the use of omalizumab in the management of chronic spontaneous urticaria in Indian patients. Indian Dermatol Online J. 2016;7(1):6.
- Société Française de Dermatologie. Consensus Conference Management of chronic urticaria Wednesday 8 January 2003 Institut Pasteur - Paris, France Recommendations. Eur J Dermatology. 2003;13(4):385–392.
- Chow SKW. Management of chronic urticaria in Asia: 2010 AADV consensus guidelines. Asia Pac Allergy. 2012;2(2):149.
- Godse K, Zawar V, Krupashankar D, Girdhar M, Kandhari S, Dhar S, et al. Consensus statement on the management of urticaria. Indian J Dermatol. 2011;56(5):485–489.
- Simons FER, Simons KJ. Histamine and H1-antihistamines: Celebrating a century of progress. Journal of Allergy and Clinical Immunology. 2011;128(6):1139–1150.
- Khakoo G, Sofianou-Katsoulis A, Perkin MR, Lack G. Clinical features and natural history of physical urticaria in children. Pediatr Allergy Immunol. 2008;19(4):363–366.
- Kjaer HF, Eller E, Høst A, Andersen KE, Bindslev-Jensen C. The prevalence of allergic diseases in an unselected group of 6-year-old children. The DARC birth cohort study. Pediatr Allergy Immunol. 2008;19(8):737–745.
- Brüske I, Standl M, Weidinger S, Klümper C, Hoffmann B, Schaaf B, et al. Epidemiology of urticaria in infants and young children in Germany - Results from the German LISAplus and GINIplus Birth Cohort Studies. Pediatr Allergy Immunol. 2014;25(1):36–42.
- Tuchinda M, Srimaruta N, Habanananda S, Vareenil J, Assatherawatts A. Urticaria in Thai children. Asian Pac J Allergy Immunol. 1986;4(1):41–45.
- Lee JY, Kang S, Park JS, Jo SJ. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean national health insurance database. Ann Dermatol. 2017;29(6):761–767.
- Choi SH, Baek HS. Approaches to the diagnosis and management of chronic urticaria in children. Korean J Pediatr. 2015;58(5):159–164.
- Chansakulporn S, Pongpreuksa S, Sangacharoenkit P, Pacharn P, Visitsunthorn N, Vichyanond P, et al. The natural history of chronic urticaria in childhood: A prospective study. J Am Acad Dermatol. 2014;71(4):663–668.
- Lee XHM, Ong LX, Cheong JYV, Sultana R, Rao R, Lim HH, et al. A stepwise approach in the management of chronic spontaneous urticaria in children. Asia Pac Allergy. 2016;6(1):16–28.
- Netchiporouk E, Sasseville D, Moreau L, Habel Y, Rahme E, Ben-Shoshan M. Evaluating comorbidities, natural history, and predictors of early resolution in a cohort of children with Chronic Urticaria. JAMA Dermatol. 2017;153(12):1236–1242.
- Pite H, Wedi B, Borrego LM, Kapp A, Raap U. Management of childhood urticaria: Current knowledge and practical recommendations. Acta Dermato-Venereologica. 2013;93(5):500–508.
- Konstantinou GN, Papadopoulos NG, Tavladaki T, Tsekoura T, Tsilimigaki A, Grattan CEH. Childhood acute urticaria in northern and southern Europe shows a similar epidemiological pattern and significant meteorological influences. Pediatr Allergy Immunol. 2011;22(1 PART 1):36–42.
- Rebelo Gomes E, Fonseca J, Araujo L, Demoly P. Drug allergy claims in children: From self-reporting to confirmed diagnosis. Clin Exp Allergy. 2008;38(1):191–198.
- Seitz CS, Bröcker EB, Trautmann A. Diagnosis of drug hypersensitivity in children and adolescents: Discrepancy between physician-based assessment and results of testing. Pediatr Allergy Immunol. 2011;22(4):405–410.
- Sackesen C, Sekerel BE, Orhan F, Kocabas CN, Tuncer A, Adalioglu G. The Etiology of Different Forms of Urticarta in Childhood. Pediatr Dermatol. 2004;21(2):102–108.
- Ricci G, Giannetti A, Belotti T, Dondi A, Bendandi B, Cipriani F, et al. Allergy is not the main trigger of urticaria in children referred to the emergency room. J Eur Acad Dermatology Venereol. 2010;24(11):1347–1348.
- Marrouche N, Grattan C. Childhood urticaria. Curr Opin Allergy Clin Immunol. 2012;12(5):485–490.
- Church MK, Weller K, Stock P, Maurer M. Chronic spontaneous urticaria in children: Itching for insight. Pediatr Allergy Immunol. 2011;22(1 PART 1):1–8.
- Jirapongsananuruk O, Pongpreuksa S, Sangacharoenkit P, Visitsunthorn N, Vichyanond P. Identification of the etiologies of chronic urticaria in children: A prospective study of 94 patients. Pediatr Allergy Immunol. 2010;21(3):508–514.
- Brunetti L, Francavilla R, Miniello VL, Platzer MH, Rizzi D, Lospalluti ML, et al. High prevalence of autoimmune urticaria in children with chronic urticaria. J Allergy Clin Immunol. 2004;114(4):922–927.
- Alangari AA, Twarog FJ, Shih MC, Schneider LC. Clinical features and anaphylaxis in children with cold urticaria. Pediatrics. 2004;113(4):e313–e317.
- Volonakis M, Katsarou-Katsari A, Stratigos J. Etiologic factors in childhood chronic urticaria. Ann Allergy. 1992;69(1):61–65.
- Du Toit G, Prescott R, Lawrence P, Johar A, Brown G, Weinberg EG, et al. Autoantibodies to the high-affinity IgE receptor in children with chronic urticaria. Ann Allergy, Asthma Immunol. 2006;96(2):341–344.
- Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155(1):145–151.
- Olsen JR, Gallacher J, Finlay AY, Piguet V, Francis NA. Quality of life impact of childhood skin conditions measured using the Children’s Dermatology Life Quality Index (CDLQI): A meta-analysis. Br J Dermatol. 2016;174(4):853–861.
- Ferrer M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergológica 2005. J Investig Allergol Clin Immunol. 2009;19(SUPPL. 2):21–26.
- Potter P, Mitha E, Barkai L, Mezei G, Santamaría E, Izquierdo I, et al. Rupatadine is effective in the treatment of chronic spontaneous urticaria in children aged 2-11 years. Pediatr Allergy Immunol. 2016;27(1):55–61.
- European Medicines Agency (EMA), European Medicines Agency, Electronic Medicines Compendium (EMC), CHMP, Electronic Medicines Compendium (EMC), European Medicines Agency (EMA), et al. Summary of product characteristics. Pharmaceutical Medicine. 2014;87–89.
- Chlorpromazine Hydrochloride. Summary of Product Characteristics. 2015. https://www.medicines.org.uk/emc/medicine/29498. Accessed 11 May 2021.
- Xyzal (oral solution) Summary of Product Characteristics. 2019. https://www.medicines.org.uk/emc/product/348/smpc#gref. Accessed 6 July 2021.
- Desloratadine (tablet) Summary of Product Characteristics. 2021. https://www.medicines.org.uk/emc/product/12005/smpc. Accessed 11 May 2021.
- Desloratadine (oral solution) Summary of Product Characteristics (SmPC) - (emc). 2020. https://www.medicines.org.uk/emc/product/7287/smpc. Accessed 6 July 2021.
- EMC. Fexofenadine hydrochloride 180mg film-coated Tablets - Summary of Product Characteristics (SmPC) - (emc). 2013. https://www.medicines.org.uk/emc/product/3488/smpc. Accessed 11 May 2021.
- Levocetirizine dihydrochloride Summary of Product Characterisitics. 2017. https://www.medicines.org.uk/emc/product/8568/smpc#gref. Accessed 6 July 2021.
- Loratadine (syrup) Summary of Product Characteristics. 2016. https://www.medicines.org.uk/emc/product/4567/smpc#gref. Accessed 6 July 2021.
- Loratadine (tablet) Summary of Product Characteristics. 2020. https://www.medicines.org.uk/emc/product/8911/smpc. Accessed 11 May 2021.
- Rupatadine (tablet) Summary of Product Characteristics. 2020. https://www.medicines.org.uk/emc/product/2501/smpc#gref. Accessed 11 May 2021.
- Rupatadine (oral solution) Summary of Product Characteristics. 2020. https://www.medicines.org.uk/emc/product/9888/smpc#gref. Accessed 6 July 2021.
- FDA. ALLEGRA® Tablets and Suspension. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021963lbl.pdf. Accessed 23 July 2021.
- Belloni Fortina A, Fontana E. Update on Antihistamine Treatment for Chronic Urticaria in Children. Current Treatment Options in Allergy. 2014;1(3):287–298.
- Corsico AG, Leonardi S, Licari A, Marseglia G, Miraglia Del Giudice M, Peroni DG, et al. Focus on the cetirizine use in clinical practice: A reappraisal 30 years later. Multidiscip Respir Med. 2019;14(1):1–7.
- Simons FER. Prevention of acute urticaria in young children with atopic dermatitis. J Allergy Clin Immunol. 2001;107(4):703–706.
- Bloom M, Staudinger H, Herron J. Safety of desloratadine syrup in children. In: Current Medical Research and Opinion. 2004. Curr Med Res Opin: 1959–1965.
- Prenner B, Ballona R, Bueso A, Cardona R, Kim K, Larsen L, et al. Safety of desloratadine syrup in children 6 months to younger than 2 years of age: A randomized, double-blinded, placebo-controlled study. Pediatr Asthma, Allergy Immunol. 2006;19(2):91–99.
- Simons FER. Safety of levocetirizine treatment in young atopic children: An 18-month study. Pediatr Allergy Immunol. 2007;18(6):535–542.
- Salmun LM, Herron JM, Banfield C, Padhi D, Lorber R, Affrime MB. The pharmacokinetics, electrocardiographic effects, and tolerability of loratadine syrup in children aged 2 to 5 years. Clin Ther. 2000;22(5):613–621.
- Doshi DR, Weinberger MM. Experience with cyclosporine in children with chronic idiopathic urticaria. Pediatr Dermatol. 2009;26(4):409–413.
- Neverman L, Weinberger M. Treatment of chronic urticaria in children with antihistamines and cyclosporine. J Allergy Clin Immunol Pract. 2014;2(4):434–438.
- Sanada S, Tanaka T, Kameyoshi Y, Hide M. The effectiveness of montelukast for the treatment of anti-histamine- resistant chronic urticaria. Arch Dermatol Res. 2005;297(3):134–138.
- Sarkar T, Sil A, Pal S, Ghosh C, Das N. Effectiveness and safety of levocetirizine 10 mg versus a combination of levocetirizine 5 mg and montelukast 10 mg in chronic urticaria resistant to levocetirizine 5 mg: A double-blind, randomized, controlled trial. Indian J Dermatol Venereol Leprol. 2017;83(5):561–568.
- Novartis Pharmaceuticals UK Ltd. Xolair 150 mg solution for injection in pre-filled syringe. 2019. https://www.medicines.org.uk/emc/product/4725/smpc. Accessed 10 May 2021.
- Cornillier H, Giraudeau B, Munck S, Hacard F, Jonville-Bera AP, d’Acremont G, et al. Chronic spontaneous urticaria in children - a systematic review on interventions and comorbidities. Pediatr Allergy Immunol. 2018;29(3):303–310.
- Goldstein S, Gabriel S, Kianifard F, Ortiz B, Skoner DP. Clinical features of adolescents with chronic idiopathic or spontaneous urticaria: Review of omalizumab clinical trials. In: Annals of Allergy, Asthma and Immunology. 2017. American College of Allergy, Asthma and Immunology: 500–504.
- Lee SJ, Ha EK, Jee HM, Lee KS, Lee SW, Kim MA, et al. Prevalence and risk factors of urticaria with a focus on chronic urticaria in children. Allergy, Asthma Immunol Res. 2017;9(3):212–219.
of interest
are looking at
saved
next event
CSU Resources and Managing CSU have been developed by EPG Health for Medthority. This content has been developed independently of the sponsor Novartis Pharma AG, who have had no editorial input into the content. EPG Health received unrestricted educational funding from the sponsor in order to help provide its healthcare professional members with access to the highest quality medical and scientific information, education and associated relevant content.
The industry-sponsored symposia and eLearning have been developed in collaboration with Novartis Pharma AG, with some content provided by Novartis Pharma AG. The views presented in the videos are those of the presenters and not necessarily those of the industry sponsor, Novartis Pharma AG. Any data on non- Novartis products are based on publicly available information at the time of content update. Prescribing information may vary depending on local health authority approval in each country. Before prescribing any product, always refer to the SmPC or product information approved in your local country.