Congress highlights
ERA 2022 congress highlights
Keep up to date with the latest in chronic kidney disease (CKD) research with our coverage of the 2022 European Renal Association (ERA) Congress held in Paris, France. Watch as professional in the field of CKD, Professor Navdeep Tangri discusses the most relevant updates to existing clinical trials and provides expert summaries of the latest research. Follow along with our written coverage of the daily congress highlights to keep your CKD clinical knowledge and practice current. Learn more about:
- Latest updates to large cohort CKD clinical trials such as DAPA-CKD and REVEAL-CKD
- Newest evidence supporting the use of sodium–glucose co-transporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists (MRAs) for people with diabetic and non-diabetic kidney disease
- Data demonstrating overlap between cardiovascular disease and type 2 diabetes in people with CKD
In this section
Congress highlights with Professor Navdeep Tangri
Watch our expert Professor Navdeep Tangri discuss research highlights from the 2022 ERA Congress. Professor Tangri focuses on the latest updates to ongoing CKD clinical trials (such as DAPA-CKD and REVEAL-CKD), the status of SGLT2 inhibitors and MRAs, as well as new research on the overlap between cardiovascular disease and type 2 diabetes in patients with CKD.
Professor Navdeep Tangri discusses key highlights from the ERA 2022 Congress including new research regarding the use of SGLT2 inhibitors in kidney transplant patients.
Professor Navdeep Tangri discusses major updates to ongoing large-cohort CKD clinical trials such as DAPA-CKD, EMPA-KIDNEY and REVEAL-CKD.
Professor Navdeep Tangri highlights the latest evidence presented at congress on the association between CKD, cardiovascular disease, and type 2 diabetes.
Professor Navdeep Tangri discusses research highlights and new insights regarding the use of SGLT2 inhibitors and MRAs for treating people with CKD.
Congress Highlights with Professor Hiddo Heerspink
Watch Professor Hiddo Heerspink discuss research highlights from the 2022 ERA Congress.
Day One Highlights – CKD diagnosis and treatment from the mini-oral sessions
By Ben Gallarda
Key chronic kidney disease developments from the mini-oral sessions
The European Renal Association (ERA) 2022 Congress began with a good number of virtual mini-oral sessions and abstracts, discussing a wide range of topics in brief online presentations. In this article, we bring you some of the key developments in chronic kidney disease (CKD) from these mini-oral sessions.
Diagnosing CKD
In a mini-oral session on lab methods, glomerular filtration rate (GFR) measurement and urine proteomics, a good part of the discussion focused on detecting CKD earlier, particularly with albuminuria testing (Figure 1). Antonio Garreta Rufas presented the results of a systematic review examining barriers that may prevent adherence to Kidney Disease Improving Global Outcomes (KDIGO) guidelines for albuminuria testing1. One of the main identified barriers was physicians’ perception that test results would not materially impact patient care. Dr Garreta Rufas concluded his presentation noting that “testing of albuminuria rates are low, and we need to improve,” along with suggestions for several ways to achieve this. These results were supported by a separate modelling study, which showed that regular urine albumin-to-creatinine ratio (UACR) testing is both cost-effective and likely to prevent more severe outcomes of CKD, including late stage disease, dialysis, and cardiovascular disease (CVD)-related deaths (Figure 1)2.
Simple and cost-effective methods exist for early detection of CKD, but are often under-utilised
Another study presented by Emilio Rodrigo asked whether routine urine analysis is appropriate for people with monoclonal gammopathy of undetermined significance (MGUS), a condition that increases in prevalence with age that may correlate with monoclonal gammopathy of renal significance (MGRS), a sign of renal damage3. Retrospective analysis of 360 patients showed that only 32% of patients with MGUS received proteinuria analysis, but that those with albuminuria or haemoglobinuria showed worse GFR. The authors conclude by recommending regular urine analysis of patient with MGUS in order to assess the potential presence of kidney damage.
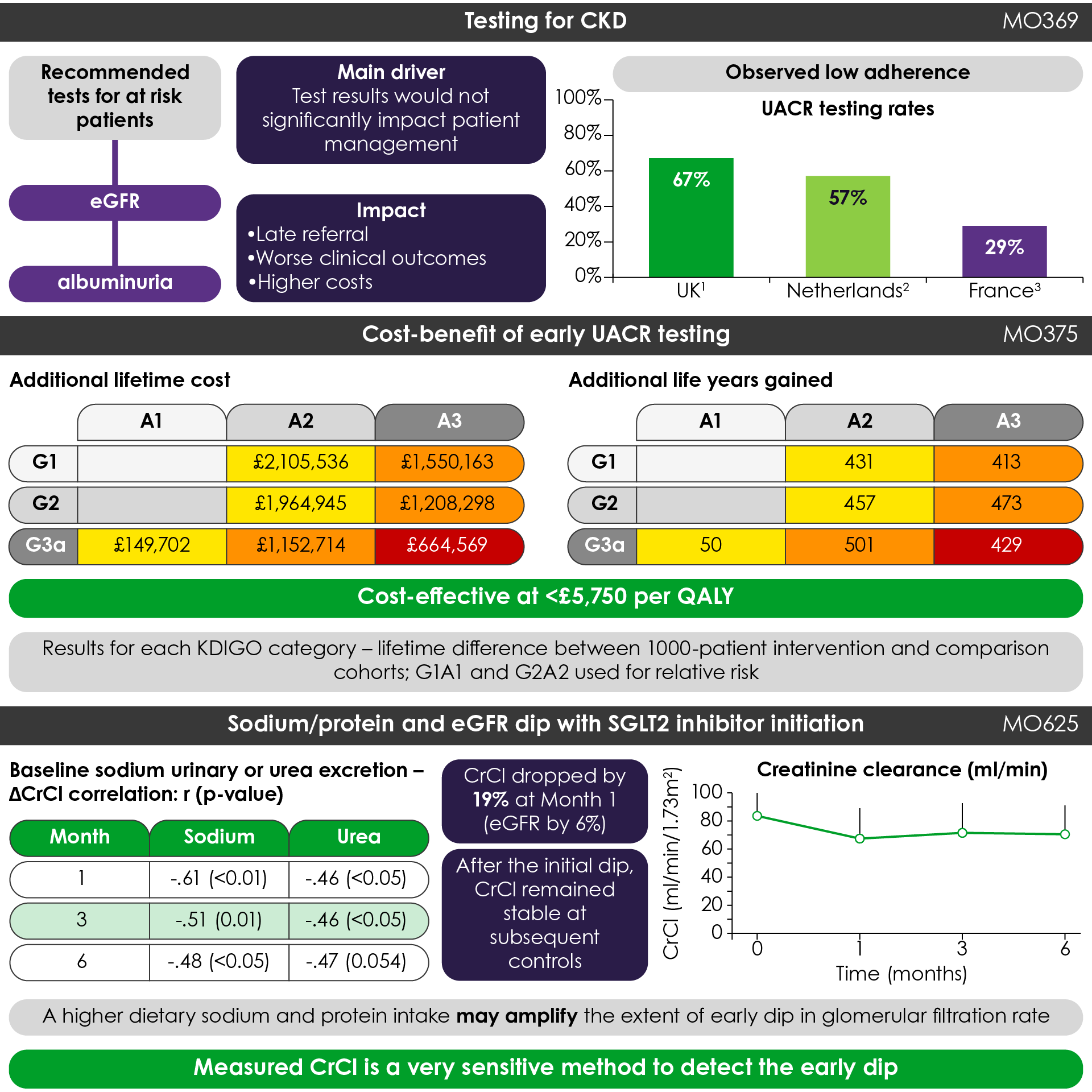
Figure 1. Key highlights from the mini-oral sessions (Adapted1,2,10). CrCl, creatinine clearance; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes; QALY, quality-adjusted life-year; SGLT2, sodium-glucose cotransporter-2; UACR, urine albumin-to-creatinine ratio.
Treating CKD
In keeping with major developments in CKD treatment in previous years, data on several sodium-glucose cotransporter 2 (SGLT2) inhibitors and a mineralocorticoid receptor antagonist were highlighted in various mini oral sessions:
- Addition of 10 mg empagliflozin daily in 30 non-diabetic patients with proteinuria significantly reduced proteinuria and blood pressure, but did not change serum creatinine levels or GFR4
- Subgroup analysis from phase 3 trials of finerenone in patients with CKD and type 2 diabetes (T2D) showed that overall positive results related to changes in UACR, estimated GFR (eGFR), and composite kidney and CV outcomes was as prominent in stage 4 CKD as in stages 1–3, with no difference in adverse events between the subgroups5
- A small, real-world study of dapagliflozin in 9 diabetic patients and 1 non-diabetic patient also receiving angiotensin-receptor blockers or angiotensin-converting enzyme inhibitors showed a preservation of kidney function and reduction in proteinuria with no documented major kidney side effects6
Promising treatments for CKD, in patients both with and without diabetes, are showing benefits in clinical trials
While SGLT2 inhibitors are showing benefits in preserving kidney function in patients withT2D, it has been documented that some patients receiving an SGTL2 inhibitor may initially see an acute decline in eGFR7-9. While this effect is typically reversible, it has the potential to affect adherence to treatment and resulting outcomes. Constanza Gaudio discussed a study examining this effect of SGLT2 inhibitor treatment in relation to high sodium and high protein intake, and measured creatinine clearance as a method to assess the extent of this eGFR dip (Figure 1)10. This study found significant correlations between high sodium and protein intake and a 6% reduction in eGFR with treatment, which were also associated with a 19% reduction in mean creatinine clearance (n = 27). Dr Gaudio concluded by noting, “A higher dietary sodium and protein intake may amplify the extent of early dip in glomerular filtration rate in diabetic patients undergoing SGLT2 inhibitor treatment,” and “measured creatinine clearance is a very sensitive method to detect this.”
One final study presented by Maria Marques Vidas looked at the safety profile of SGLT2 inhibitors in patients 75 years of age or older (75+, n = 33) and found no increase in the rate of side effects when compared to younger patients (<75, n = 120). The most common side effect in both groups was genitourinary tracts infections in 10.3% of all patients, which were more common in the first year of treatment11.
Day Two Highlights – From late-breaking trials to CKD classification
By Anita Reid
Late-breaking clinical trials
Several late-breaking clinical summaries were revealed on the second day of the European Renal Association (ERA) 2022 Congress.
Patrick Rossignol reported that “all endpoints were statistically and convincingly” met in the DIAMOND Trial12 investigating patiromer, a novel K+ binder, in patients with comorbid heart failure (HF) with reduced ejection fraction and chronic kidney disease (CKD), and in whom hyperkalaemia leads to compromised treatment with renin-angiotensin-aldosterone system inhibitors (RAASi)13. Patiromer maintained lower serum K+ levels vs placebo across all CKD subgroups and resulted in a lower incidence of hyperkalaemic events, as well as a greater proportion of patients being maintained on a mineralocorticoid receptor antagonist (MRA) at target doses vs placebo across all CKD groups.
K+ binder, patiromer, reduces incidence of hyperkalaemic events in CKD with HF
The tubular stress biomarker, urinary dickkopf-3 (uDKK3), is a predictor of acute kidney injury (AKI) risk and chronic kidney disease (CKD) progression in adults14-16. According to Franz Schaefer and colleagues’ post-hoc analysis of the 4CS17 and ESCAPE trials18, higher levels of uDKK3 were associated with faster loss of estimated glomerular filtration rate (eGFR) in paediatric CKD. This was independent of underlying disease, age, baseline eGFR, proteinuria, hypertension and obesity. It was only in patients with high uDKK3 levels that RAAS inhibition and intensified blood pressure control were associated with reduced eGFR loss. “UDKK3 is a promising new biomarker that may be useful in predicting responsiveness to nephroprotective therapies and designing risk-adapted personalised medicine,” said Dr Schaefer.

Classification of CKD
The classification of CKD continues to be challenged; however a study by the UK Biobank cautions against age-related thresholds for CKD diagnosis in older people19.
Do age-related thresholds overlook the risk of CV disease associated with CKD?
Age-related thresholds have been proposed for CKD diagnosis20 to reflect the normal age-related decline in renal function over the age of 40 years, by raising the existing threshold for CKD under 40 years and reducing it over 65 years19. However, as Jennifer Lees pointed out: “The primary risk associated with CKD is cardiovascular (CV) disease; lowering the threshold may miss this risk in this [elderly] group”. Dr Lees was reporting a UK Biobank study that showed cystatin C testing for eGFR (eGFRcys) detected a substantial number of high-risk individuals not identified as having CKD by creatinine testing (eGFRcr). Lower, age-adapted thresholds for CKD diagnosis, may therefore be inadequate at detecting broader risks associated with CKD in older people.

Progression in diabetic kidney disease
A symposium on diabetic kidney disease (DKD) included progress in the early detection of DKD and new emerging treatments.
In the first presentation, Gove Spasovski reviewed progress in earlier (molecular) detection of DKD with proteonomics, including the prediction of microalbuminuria and eGFR decline in the clinical trial, PRIORITY21, 22. Disappointingly, subsequent early intervention with aldosterone blocker, spirolactone, did not delay progression of early diabetic nephropathy vs placebo23. Dr Spasovski also reviewed guidance on treatment recommendations in DKD:
- Use SGLT2 inhibitors whenever possible in type 2 diabetes (T2D) (eGFR > 30 ml/min/1.73 m2) to reduce the risk of CKD and cardiorenal outcomes, as shown in CREDENCE and other studies, including a meta-analysis24-26. In addition, SLGT-2 inhibitors have protective effects beyond glycaemic control for the kidneys, heart and other organs27, including antioxidative effects28
- Use GLP-1 receptor agonists in advanced CKD (eGFR <30 ml/min/1.73m2) and HF, as shown by a systematic review and meta-analysis of CV outcome trials29
- Add a MRA (finerenone) to standard care in CKD with HF: in the FIDELIO-DKD study, finerenone vs placebo protected against CV events and kidney disease progression in patients with T2D and early- or late-stage CKD, irrespective of baseline HF30.
SGLT2 inhibitors should be used whenever possible in T2D to reduce the risk of CKD and negative cardiorenal outcomes
Is AKI preventable in CKD? Arici Mustafa explored this question by first highlighting that CKD is the leading cause of AKI, and that AKI directly exacerbates CKD progression31. Interrupting this cycle is therefore of clinical significance. While the use of the nephrology rapid response team (NRRT) has proven to be effective in preventing AKI32, novel strategies are still under investigation. One such strategy is the use of automated e-alerts based on static patient data, however, in numerous, large cohort, multi-centre studies, no significant improvements in AKI prevention were observed33. Another strategy that is currently in development is the use of artificial intelligence (AI) to create individual prediction models using baseline and evolving clinical data collected during hospital admission. Studies investigating AKI risk prediction using AI have demonstrated improved patient outcomes, however the technology still needs to be optimised and developed in a way that can be easily implemented in clinic34. Dr Arici concluded by noting that until AI risk-prediction becomes clinically reliable, the most effective method of AKI prevention is the NRRT.
Day Three Highlights – Patient experience of COVID and treatment studies in CKD
By Anita Reid
Patient experience during COVID
A patient association presented for the first time at a European Renal Association Congress.
Yvanie Caillé, founder of the French patient association, ‘Renaloo’, and eminent sociologist and vice-chair of Renaloo, Christian Baudelot, met virtually with prominent nephrologist, Raymond Vanholder, chair of the Brussel-based European Kidney Health Alliance. Patient associations were praised for their activity during the COVID-19 crisis.
The time has come to place a higher value on the role of patients’ associations. Increasingly, these associations are supporting patients and provide valuable insight.
From the results of Renaloo’s latest patient survey, Yvanie Caillé highlighted poor vaccine coverage with 57% of dialysis patients and 54.7% kidney transplant recipients receiving their first booster vs 82% in the eligible population, and severe underuse of COVID-19-prophylactic, monoclonal antibody therapy, tixagevimab/cilgavimab (Evusheld).
57% of dialysis patients and 54.7% of kidney transplant patients have had their first booster vs 82% of the eligible population
Professor Vanholder, called for urgent action in the ‘Decade of the Kidney’ (2020-30). Although chronic kidney disease (CKD) affects nearly 100 million Europeans, kidney disease is absent from the list of European Union (EU) key health research areas, and many people with kidney disease are diagnosed late35.
Treatment for chronic kidney disease
A post-hoc analysis of the DAPA-CKD trial is presented, while finerenone and canaglifozin are compared in the absence of head-to-head trials.
In the DAPA-CKD trial, the sodium-glucose cotransporter 2 inhibitor (SGLT2i), dapagliflozin, reduced the risk of kidney failure in patients with chronic kidney disease (CKD)36,37. Hiddo Heerspink reported the post-hoc analysis of DAPA-CKD assessing the efficacy of dapagliflozin by baseline dosage of angiotensin-converting enzyme inhibitor (ACEi) or angiotensin-receptor blocker (ARB) in reducing major adverse cardiorenal outcomes in CKD. The benefit of dapagliflozin was consistent for primary outcome (defined as at least 50% sustained decline in estimated glomerular filtration rate [eGFR], end-stage renal disease, or death from renal or cardiovascular [CV] causes), and secondary outcomes across the background dosage of ACEi or ARB. Of 4296 participants (99.9% of the study population) with available data on ACEi/ARB doses, 1231 (28.7%) were using the target dose, 1867 (43.5%) a dose ≥ 50% to <100% of target and 1068 (24.9%) a dose 0 to <50% of target, while 130 (3.0%) were not using an ACEi/ARB. The event rate for the primary outcome was highest among participants not using ACEi/ARBs vs other subgroups. Dapaglifozin slowed the rate of eGFR decline vs placebo over the duration of the study by –0.93 mL/min/1.73 m2 (95% CI, 0.61–1.25], irrespective of the dosage of ACEIs or ARBs (p value for interaction 0.877).
Dapaglifozin slows eGFR decline irrespective of antihypertensive medication
Both finerenone (mineralocorticoid-receptor antagonist [MRA]) in FIDELIO-DKD and canagliflozin (SGLT2i) in CREDENCE reduced cardiovascular and kidney disease vs placebo in patients with CKD and type 2 diabetes, on top of renin-angiotensin-aldosterone system blockage38-40. Due to the lack of direct comparison between finerenone and canagliflozin, David Cherney described the use of individual patient weighting, using matching-adjusted indirect comparison (MAIC) methodology and placebo as a common comparator, to adjust for differences between the trials in selected baseline characteristics and primary outcomes. As anticipated, finerenone showed a higher risk of hyperkalaemia; otherwise, there was no evidence of significant treatment difference between finerenone and canalglifloxin.
Clinical outcomes in diabetic kidney disease and CV complications
Key trials in semaglutide are highlighted and the link between vitamin D levels and MMP-10.
Vlado Perkovic presented the blinded baseline characteristics of the FLOW study population41. FLOW is a kidney outcomes trial designed to evaluate the effect of once-weekly semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA), in individuals with type 2 diabetes (T2D) and CKD42. GLP-1RAs lower blood glucose but also reduce risk of MACE43-45 and have potential direct kidney-protective effects46,47. Other key clinical trials on semaglutide in CKD, T2D, obesity and CVD include:
- REMODEL (NCT04865770) – subcutaneous (sc) semaglutide, 1.0 mg48
- SELECT (NCT03574597) – sc semaglutide, 2.4 mg49
- SOUL (NCT03914326) – oral semaglutide, 1.9 mg50
Vitamin D levels are inversely correlated with metalloproteinase-10 levels in T2D
Daria Abasheva reported a cross-sectional study (n = 256) that found an inverse association between circulating levels of 25-hydroxyvitamin D and MMP-10 in T2D, especially with CKD and at the extreme of vitamin D range (>45 ng/ml), independent of eGFR. Vitamin D deficit was significantly more prevalent with CKD (61%) than without CKD (40%) (p<0.001)51.
Day Four Highlights – The pillars of therapy to reduce cardio renal risk
By Anita Reid
During the last day of the ERA 2022 conference, there was a thorough discussion of the FIDELITY study in patients with CKD and type 2 diabetes, which called for a holistic approach to treatment.
Clinicians now have the benefit of add-on treatment with a new class of non-steroidal mineralocorticoid receptor antagonists
Individuals with type 2 diabetes (T2D) have a strong residual risk of chronic kidney disease (CKD) progression, despite being treated with current therapies, i.e., angiotensin-converting enzyme inhibitors (ACEi), angiotensin-receptor blockers (ARBs) and the sodium-glucose cotransporter 2 inhibitors (SGLT2i)52.
However, clinicians now have the benefit of add-on treatment with a new class of non-steroidal mineralocorticoid receptor antagonists (MRA), specifically finerenone, which has been the most extensively studied agent within the class to date, with pending results from glucagon-like peptide 1 receptor agonists (GLP1-RA) also showing promising results as add-on treatment.
Katherine Tuttle emphasised the importance of precise phenotyping, even based on the patient’s clinical picture, to improve the selection of therapies and how to apply them for therapeutic safety and efficacy.
George Bakris reviewed the results of FIDELITY, a large, pooled analysis of individual patient data (N=13171) taken from FIDELIO-DKD and FIGARO-DKD53,54. Both trials investigated the effect of finerenone on kidney and cardiovascular (CV) outcomes in patients with CKD and T2D.
FIDELITY showed that, on top of standard care (ACEIs/ARBs), finerenone reduced the risk of clinically meaningful CV and kidney outcomes in patients with T2D over a broad spectrum of CKD:
- endpoint CV composite: 14% (HR 0.86; 95% CI, 0.78–0.95; P=0.0018)
- hospitalisation for heart failure (HHF): 22% (HR 0.78; 95% CI, 0.66–0.92; P=0.0030)
- kidney composite: 23% (HR 0.77; 95% CI, 0.77–0.88; P=0.0002)
- dialysis: 20% (HR 0.80; 0.64–0.99; P=0.040) In addition to standard care, finerenone demonstrated CV benefits and preservation of kidney function in patients with T2D, regardless of the baseline estimated glomerular filtration rate (eGFR) or urinary albumin:creatinine ratio (UACR) and the use of SGLT2s or GLP-1RAs, with benefit primarily driven by reduction in HHF. Finerenone showed modest effects on systolic blood pressure and no sexual side-effects. Although hyperkalaemia increased with finerenone, the clinical impact was low; interestingly, for yet unknown reasons, hyperkalaemia risk is decreased in the presence of SGLT2is53
Dr Bakris asked nephrologists to think like cardiologists about treatment, inviting them to think of ‘pillars of therapy’ rather than drug A vs drug B, or fixing sugar or fixing blood pressure. He described the pillars of therapy to reduce cardio renal risk: firstly, maximally tolerated doses of ACE inhibitors and ARBs, secondly, SGLT2i, and now finerenone, a non-steroidal MRA.

The pooling of data in FIDELITY, from the FIDELIO-DKD and FIGARO-DKD trials, has provided a large group of more than 13000 patients53. Subgroup analyses enables the use of finerenone to be refined in subgroups of patients with CKD and T2D. Peter Rossing concluded that in all the subgroups studied, whether in albuminuria, eGFR, presence of CV disease defined in different ways, different levels of blood pressure, different levels of glycaemic control, whether on GLP-1RA or SGLT2i therapy, very consistent kidney and cardio benefits of finerenone compared with placebo were observed. Dr Rossing summarised the key subgroup analyses performed so far from FIDELITY; further analysis is still being completed.
- Based on kidney outcomes (albuminuria), finerenone helps protect against CV events and kidney disease progression; the CV outcome is consistent for micro- or macroalbuminuria
- Using a different renal endpoint, i.e., slope in eGFR, finerenone shows a benefit in all patients
- In different subgroups of CV disease (myocardial infarction [MI] and/or ischaemic stroke, coronary artery disease, peripheral artery disease), finerenone consistently reduced the risk of CV events in all these subgroups; in addition, the risk of CV events was reduced, with or without a history of a CV event
- Modest reduction in blood pressure vs placebo occurred across baseline blood pressure56
Diabetes is responsible for approximately 50% of all cases of CKD and kidney failure or end-stage kidney disease (ESKD) worldwide57; indeed in Katherine Tuttle’s experience, nephrologists take care of more diabetic patients than endocrinologists, while most diabetes-associated cardiovascular disease occurs in those with CKD. The latest KDIGO guideline recommends a holistic approach for these patients using a portfolio of therapies to personalise treatment58. Dr Tuttle reviewed the safety data for finerenone and how to individualise it for individuals with diabetes and CKD. As an add-on to first-line therapy, Dr Tuttle stated that finerenone is for patients who have persistently low albuminuria and normal potassium to protect both the heart and the kidney in these individuals.
Finerenone is not a fire and forget medicine. For safe administration it requires monitoring and careful attention to both GFR and K+
Dr Tuttle addressed the risk of hyperkalaemia, which has been the main limitation to finerenone and other MRA inhibitors. Several predictive factors for hyperkalaemia were identified by the FIDELIO-DKD study53, especially baseline K+ >4.5 mEq/L or a low eGFR <45 mg/ml/1.73m2 and the use of betablockers, but mitigating factors, SGLT2i or diuretic, were also identified.
Dr Tuttle reviewed a prescribing protocol to safely individualise finerenone prescribing by adjusting for both eGFR and serum K+ levels. She emphasised the importance of follow-up eGFR and K+ monitoring and adjustment of dosage.
This is something that nephrologists are going to have to learn to do. This is not a fire and forget medicine. It does require monitoring and careful attention to both GFR and K+ to safely administer finerenone.
Chronic kidney disease affects approximately 11% of the global population, yet remains under-diagnosed, especially in the early stages59
This statistic formed the basis of Dr De Nicolas’ presentation of the REVEAL-CKD study investigating the prevalence of undiagnosed stage 3 CKD in the global population. Dr De Nicolas’ presentation focused on preliminary data of undiagnosed stage 3 CKD in an Italian population which recruited 65,676 patients, 15,129 of which had an existing CKD diagnosis and 50,547 without60, Of the undiagnosed group, 15% were found to have CKD, with median diagnosis time taking 1.1 years. Further analysis revealed that among this group, 77% had undiagnosed stage 3 CKD, with the chances of misdiagnosis increasing if the patients were female, >65 and had comorbidities. Dr De Nicola concluded his presentation by noting that undiagnosed CKD is a critical problem. He emphasised that timely diagnosis is vitally important for reducing progression and complications and should be a central focus for all treating physicians61.
References
- Garreta Rufas A, Meredith K, Harris J, Rossing P, Hobbs R, Wanner C. A systematic review to evaluate the drivers of non-adherence to albuminuria testing guidelines and the clinical and economic impact of not Identifying CKD over the course of progressive kidney function loss. Presented at the ERA Congress 2022, 19 May. Paris. MO369.
- Mernagh P, Folkerts K, Rufas AG, Meredith K, Harris J, Wanner C, et al. The health economic impact of accurately diagnosing CKD with and without a UACR test at different points in the pathway of disease progression. Presented at the ERA Congress 2022, 19 May. Paris. MO375.
- Iturralde Ros M, Maiztegi Azpitarte A, Ortiz Espejo M, Montes Gaisan C, María Ocio San Miguel E, Ruiz San Millán JC, et al. Should we systematically look for urine alterations in all patients with monoclonal gammopathy of undetermined significance? Presented at the ERA Congress 2022, 19 May. Paris. MO371.
- Afrooghe A, Nematollahi N, Sadat Hakemi M, Nassiri A. Evaluating the effect of SGLT2inhibitors on proteinuria and glomerular filtration rate in non-diabetic patients with proteinuria. Presented at the ERA Congress 2022, 19 May. Paris. MO157.
- Sarafidis P, Ruilope L, Anker SD, Agarwal R, Pitt B, Filippatos G, et al. Outcomes with finerenone in patients with stage 4 chronic kidney disease and type 2 diabetes: a FIDELITY subgroup analysis. Presented at the ERA Congress 2022, 19 May. Paris. MO198.
- Griveas I. Dapagliflozin and kidney outcomes in CKD patients with and without type 2 diabetes: real life data (precursor announcement). Presented at the ERA Congress 2022, 19 May. Paris. MO387.
- Adamson C, Docherty KF, Heerspink HJL, de Boer RA, Damman K, Inzucchi SE, et al. Initial Decline ("dip") in Estimated Glomerular Filtration Rate Following Initiation of Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction: Insights from DAPA-HF. Circulation. 2022.
- Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, et al. Characterization and implications of the initial estimated glomerular filtration rate 'dip' upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021;99(3):750-762.
- Heerspink HJL, Cherney DZI. Clinical Implications of an Acute Dip in eGFR after SGLT2 Inhibitor Initiation. Clinical Journal of the American Society of Nephrology. 2021;16(8):1278-1280.
- Gaudio C, Colombi C, Merciai C, Seghieri M, Spatoliatore G, Rosati A. Effects of dietary sodium and protein intake on early dip of GFR levels in subjects with type 2 diabetes treated with SGLT2 inhibitors. Presented at the ERA Congress 2022, 19 May. Paris. MO625.
- Marques Vidas M, Domenech E, Martin Testillano L, Maroto A, Lopez P, Portoles JM. Safety of SGLT2 inhibitors (SGLT2i) in people over 75 years. Presented at the ERA Congress 2022, 19 May. Paris. MO167.
- Butler J, Anker SD, Siddiqi TJ, Coats AJS, Dorigotti F, Filippatos G, et al. Patiromer for the management of hyperkalaemia in patients receiving renin-angiotensin-aldosterone system inhibitors for heart failure: design and rationale of the DIAMOND trial. Eur J Heart Fail. 2022;24(1):230-238.
- Rossignol P. Patiromer for the management of hyperkalemia in heart failure patients with reduced ejection fraction receiving renin-angiotensin-aldosterone system inhibitors: results from a prespecified analysis by eGFR from the DIAMOND trial. Presented at the ERA Congress 2022, 20 May. Paris. S 0.1.
- Schunk SJ, Zarbock A, Meersch M, Kullmar M, Kellum JA, Schmit D, et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet. 2019;394(10197):488-496.
- Zewinger S, Rauen T, Rudnicki M, Federico G, Wagner M, Triem S, et al. Dickkopf-3 (DKK3) in Urine Identifies Patients with Short-Term Risk of eGFR Loss. J Am Soc Nephrol. 2018;29(11):2722-2733.
- Schaefer F. Urinary DKK3 predicts short-term EGFR decline and nephroprotective efficacy of antihypertensive therapy in children with CKD. Presented at the ERA Congress 2022, 20 May. Paris. S 0.1.
- Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, et al. Cardiovascular Phenotypes in Children with CKD: The 4C Study. Clin J Am Soc Nephrol. 2017;12(1):19-28.
- Siragy HM. ESCAPE: From hypertension to renal failure. Curr Hypertens Rep. 2010;12(4):207-209.
- Lees J. Cystatin C testing improves risk stratification associated with chronic kidney disease without adopting age-adapted diagonstic thresholds: A UK Biobank study. Presented at the ERA Congress 2022, 20 May. FC 077.
- Delanaye P, Jager KJ, Bökenkamp A, Christensson A, Dubourg L, Eriksen BO, et al. CKD: A call for an age-adapted definition. Journal of the American Society of Nephrology. 2019;30(10):1785-1805.
- Lindhardt M, Persson F, Currie G, Pontillo C, Beige J, Delles C, et al. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): essential study design and rationale of a randomised clinical multicentre trial. BMJ Open. 2016;6(3):e010310.
- Spasovski G. Diabetic kidney disease - early detection and subsequent intervention for delaying the progression. Presented at the ERA Congress 2022, 20 May. Paris. S 4.8.
- Tofte N, Lindhardt M, Adamova K, Bakker SJL, Beige J, Beulens JWJ, et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8(4):301-312.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine. 2019;380(24):2295-2306.
- Gilbert RE, Thorpe KE. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: A meta-analysis of cardiovascular outcome trials. Diabetes Obes Metab. 2019;21(8):1996-2000.
- Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845-854.
- Tuttle KR, Brosius FC, 3rd, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, et al. SGLT2 Inhibition for CKD and Cardiovascular Disease in Type 2 Diabetes: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Diabetes. 2021;70(1):1-16.
- Tsai KF, Chen YL, Chiou TT, Chu TH, Li LC, Ng HY, et al. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants (Basel). 2021;10(8).
- Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776-785.
- Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474-484.
- Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101-107.
- Rizo-Topete LM, Rosner MH, Ronco C. Acute Kidney Injury Risk Assessment and the Nephrology Rapid Response Team. Blood Purif. 2017;43(1-3):82-88.
- Baird D, De Souza N, Logan R, Walker H, Guthrie B, Bell S. Impact of electronic alerts for acute kidney injury on patient outcomes: interrupted time-series analysis of population cohort data. Clin Kidney J. 2021;14(2):639-646.
- Mistry NS, Koyner JL. Artificial Intelligence in Acute Kidney Injury: From Static to Dynamic Models. Adv Chronic Kidney Dis. 2021;28(1):74-82.
- Vanholder R, Annemans L, Bello AK, Bikbov B, Gallego D, Gansevoort RT, et al. Fighting the unbearable lightness of neglecting kidney health: the decade of the kidney. Clinical Kidney Journal. 2021;14(7):1719-1730.
- Lambers Heerspink H, Jong N, Stefansson B, Chertow G, Maria Langkilde A, McMurray J, et al. FC082: Effects of Dapagliflozin in Patients with Chronic Kidney Disease According to Background Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Dose. Nephrology Dialysis Transplantation. 2022;37(Supplement_3).
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in Patients with Chronic Kidney Disease. New England Journal of Medicine. 2020;383(15):1436-1446.
- Cherney D, Folkerts K, Mernagh P, Nikodem M, Pawlitschko J, Rossing P. FC083: Finerenone and Canagliflozin in the Treatment of Chronic Kidney Disease and Type 2 Diabetes: Matching-Adjusted Indirect Treatment Comparison of Fidelio-DKD and Credence. Nephrology Dialysis Transplantation. 2022;37(Supplement_3).
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. New England Journal of Medicine. 2020;383(23):2219-2229.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295-2306.
- Perkovic V, Baeres F, Bakris G, Bosch-Traberg H, Idorn T, Mahaffey K, et al. FC 123: Baseline Characteristics of the Flow Trial Population: Kidney Outcomes Trial With Once-Weekly Semaglutide in People With Type 2 Diabetes and Chronic Kidney Disease. Nephrology Dialysis Transplantation. 2022;37(Supplement_3).
- NCT03819153. A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03819153. Accessed 25 May 2022.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. New England Journal of Medicine. 2016;375(19):1834-1844.
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. Drug and Therapeutics Bulletin. 2016;54(9):101-101.
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet. 2019;394(10193):121-130.
- Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and Renal Outcomes in Type 2 Diabetes. New England Journal of Medicine. 2017;377(9):839-848.
- Shaman AM, Bain SC, Bakris GL, Buse JB, Idorn T, Mahaffey KW, et al. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145(8):575-585.
- NCT04865770. A Research Study to Find Out How Semaglutide Works in the Kidneys Compared to Placebo, in People With Type 2 Diabetes and Chronic Kidney Disease (the REMODEL Trial). Available at: https://clinicaltrials.gov/ct2/show/NCT04865770. Accessed 25 May 2022.
- NCT03574597. Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity. Available at: https://clinicaltrials.gov/ct2/show/NCT03574597. Accessed 25 May 2022.
- NCT03914326. A Heart Disease Study of Semaglutide in Patients With Type 2 Diabetes. Available at: https://clinicaltrials.gov/ct2/show/NCT03914326. Accessed 25 May 2022.
- Abasheva D, Marta D, Fernandez-Seara MA, Maria Mora Gutierrez J, Antonio Rodriguez J, Javier Escalada F, et al. FC 126: Association Between Circulating Levels of 25-Hydroxyvitamin D and Metalloproteinase-10 (MMP 10) in Patients With Type 2 Diabetes Mellitus. Cross-Sectional Study. Nephrology Dialysis Transplantation. 2022;37(Supplement_3).
- Bakris G. FIDELITY - Cardiorenal outcomes across the spectrum of CKD in T2D. Presented at the ERA Congress 2022, 22 May. Paris. S 4.10.
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. New England Journal of Medicine. 2020;383(23):2219-2229.
- Luis, Agarwal R, Stefan, George, Filippatos G, Nowack C, et al. Design and Baseline Characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease Trial. American Journal of Nephrology. 2019;50(5):345-356.
- Filippatos G. Abstract 7161. Finerenone benefits patients with diabetes across spectrum of kidney disease. Presented at the ESC Congress 2021. Available at: https://www.escardio.org/The-ESC/Press-Office/Press-releases/Finerenone-benefits-patients-with-diabetes-across-spectrum-of-kidney-disease.
- Rossing, P. Implications of key subgroup analyses from the trial programme. Presented at the ERA Congress 2022, 22 May. Paris. S 4.10.
- Tuttle, K. Safety and considerations for optimising patient outcomes. Presented at the ERA Congress 2022, 22 May. Paris. S 4.10.
- Clinical Practice Guidelines for Diabetes Management in Chronic Kidney Disease. KDIGO. 2022.
- Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Nephrology Dialysis Transplantation. 2019;34(11):1803-1805.
- De Nicola L, Peach E, Barone S, Ripellino C, Heiman F, Tangri N. REVEAL-CKD: Prevalence of undiagnosed stage 3 chronic kidney disease in Italy. Presented at the ERA Congress 2022, 19 May. Paris. M0509.
- Levin A, Stevens PE. Early detection of CKD: the benefits, limitations and effects on prognosis. Nat Rev Nephrol. 2011;7(8):446-457.
of interest
are looking at
saved
next event
This content has been developed independently by Medthority who previously received educational funding in order to help provide its healthcare professional members with access to the highest quality medical and scientific information, education and associated relevant content.